Oral solid preparation for treating bladder cancer and preparation method of oral solid preparation
A solid and tablet technology, applied in the direction of non-active ingredient medical preparations, pill delivery, pharmaceutical formulations, etc., can solve the problems that diabetic patients are not suitable for long-term use, complicated process steps, difficult quality control, etc., and achieve good clinical use Value and social benefits, simple preparation process, and the effect of avoiding burst release phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The preparation one of embodiment 1 nitroxyquinoline oral solid tablet core
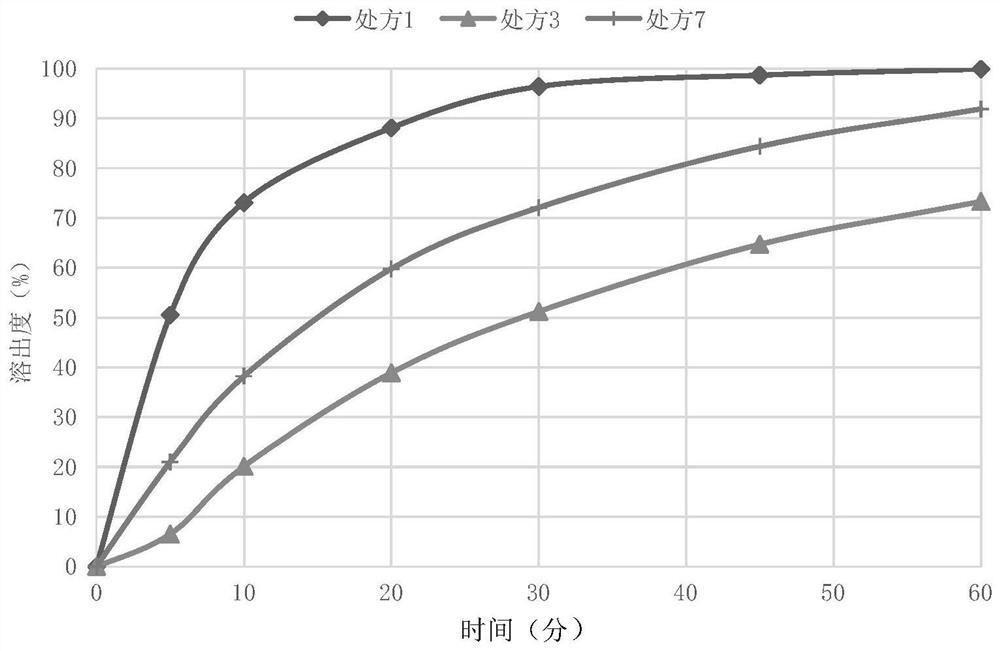
[0058] In order to obtain a tablet core prescription with a moderate dissolution rate and avoid sudden release, the types and proportions of excipients are selected. Based on the total weight of the tablet core, the weight percentages of various components are shown in Table 1 below.
[0059] The percentage by weight of various components in each prescription of table 1
[0060]
[0061] The preparation method of nitroxyquinoline tablet tablet core:
[0062] (1) according to the above table, take respectively nitroquinoline, filler, disintegrating agent, binding agent and lubricant of each prescription amount;
[0063] (2) 10 mesh sieves are crossed by nitraxine, and 60 mesh sieves are crossed by other components, for subsequent use;
[0064] (3) the binder (starch) of each prescription amount is mixed with the aqueous solution of 5% mass concentration with purified water, for subsequent...
Embodiment 3
[0072] The preparation two of embodiment 3 nitroxyquinoline oral solid tablet core
[0073] Taking the above-mentioned prescription 7 with a moderate release rate as a sample, after the preliminary influencing factor test at 60°C for 10 days (the tablet core was placed in a blast drying oven at 60°C for 10 days), it was found that the tablet could hardly disintegrate. After analysis and research, it is found that the reason may be that the nitroxoquinoline as an ion complexing agent has taken away the magnesium in the magnesium stearate, so that the stearic acid is freed, and the stearic acid with a low melting point and hydrophobicity is at the accelerated test temperature. The water-permeable channels in the sheet are blocked after melting, causing the disintegration rate to decrease. In order to solve the problem of aging of prescriptions containing magnesium stearate, lubricants were screened, and stearic acid was used as a positive control formula (prescription 8) for ver...
Embodiment 5
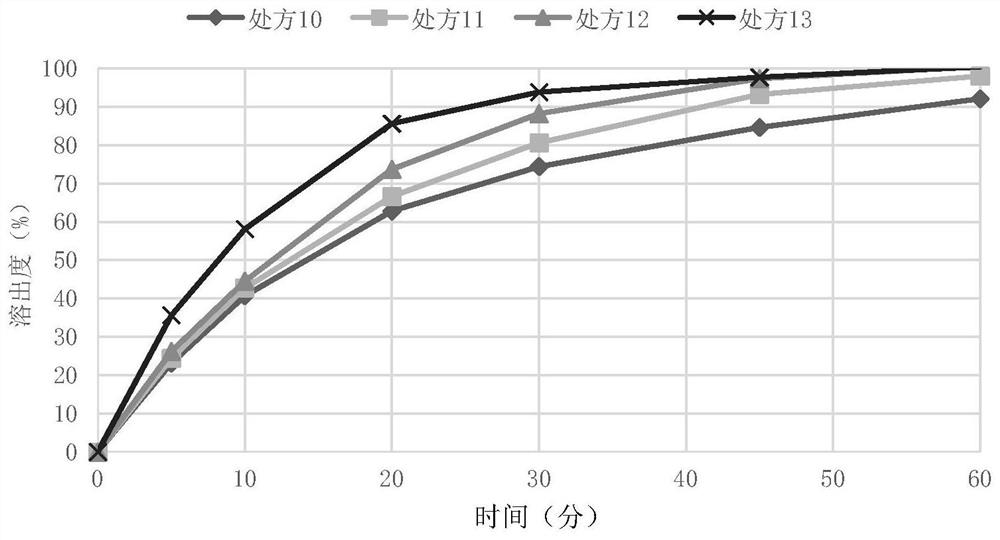
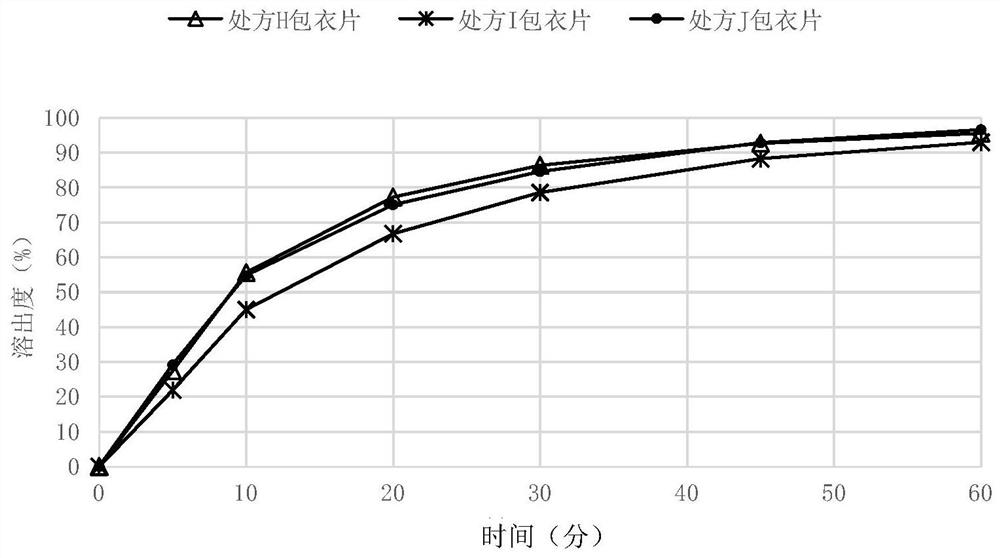
[0088] Embodiment 5 nitroxyquinoline oral solid tablet core and its preparation
[0089] Based on the total weight of the tablet core, the weight percentages of various components are shown in Table 3 below. Wherein the binder formula ratio is the proportion of solids used, and the binder used in the preparation is an aqueous solution of 5% starch.
[0090]
[0091] Preparation:
[0092] (1) according to the above table, take respectively nitroquinoline, filler, disintegrating agent, binding agent and lubricant of each prescription amount;
[0093] (2) 10 mesh sieves are crossed by nitraxine, and 60 mesh sieves are crossed by other components, for subsequent use;
[0094] (3) The binder (starch or hypromellose) is prepared into an aqueous solution of 5% mass concentration with purified water for subsequent use;
[0095] (4) Nitroxine, filler (lactose, starch, microcrystalline cellulose) and disintegrant (hydroxypropyl cellulose) of each prescription amount are put into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com