Method for synthesizing herbicide pyriminobac-methyl in paddy field
A synthesis method and a technology for pyrimifen, applied in the field of synthesizing the paddy field herbicide pyrimifen, can solve the problems of difficult industrialized production, unfavorable industrialized production, low reaction yield and the like, and achieve easy industrialized production and low production cost. , The effect of simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
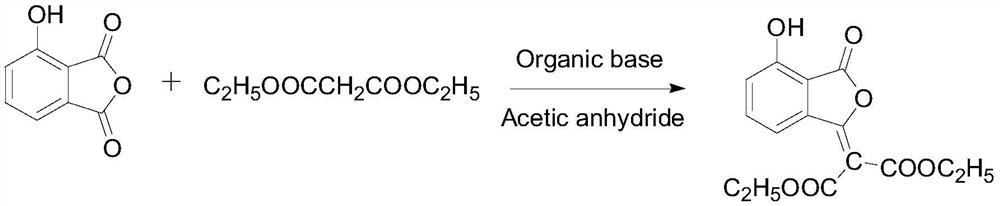
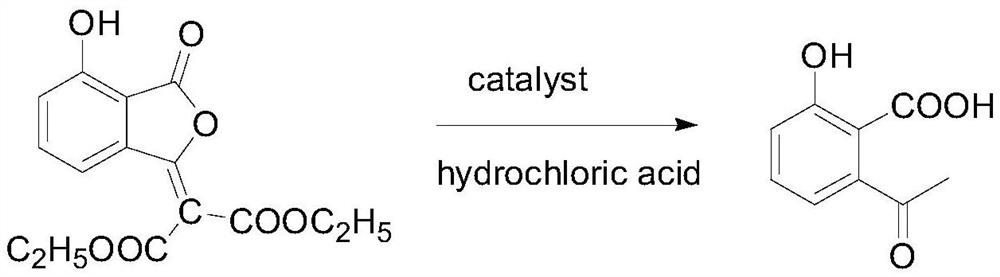
Examples
Embodiment 1
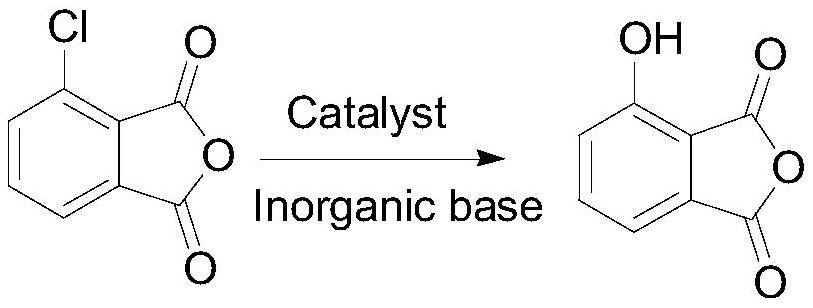
[0044] a. Preparation of 3-hydroxyphthalic anhydride
[0045] Add 3-chlorophthalic anhydride (22 grams, 120.5mmol), N,N-dimethylformamide (60mL), water (30mL) and catalyst cuprous chloride (2.38 grams, 24mmol) in the reaction flask, stir , add potassium hydroxide (27.04 g, 482 mmol), and react at 115°C for 12 hours. After the reaction, filter with suction, wash the filter cake with dichloromethane, adjust the pH value of the filtrate to 1~2, extract with dichloromethane, and collect the organic solvent And concentrated to obtain 22.1 grams of reddish-brown oily liquid 3-hydroxyphthalic acid, HPLC: 88%;
[0046] Add the above-mentioned 3-hydroxyphthalic acid (22.1 grams, HPLC: 88%) in the reaction flask, add acetic anhydride (36.8 grams, 361.5mmol), stir and heat to 80 ° C for 2 hours, after the reaction, the reaction solution decreases Concentrate under reduced pressure to obtain 18.2 g of reddish-brown oily liquid 3-hydroxyphthalic anhydride, with a yield of 80.1%.
[0047]...
Embodiment 2
[0064] a. Preparation of 3-hydroxyphthalic anhydride
[0065] Add 3-chlorophthalic anhydride (22 grams, 120.5mmol), N,N-dimethylformamide (60mL), water (30mL) and catalyst cuprous chloride (2.38 grams, 24mmol) in the reaction flask, stir , add potassium hydroxide (40.5 g, 723 mmol), and react at 105°C for 12 hours. After the reaction, filter with suction, wash the filter cake with dichloromethane, adjust the pH value of the filtrate to 1~2, extract with dichloromethane, and collect the organic solvent And concentrated to obtain 24.1 grams of reddish-brown oily liquid 3-hydroxyphthalic acid, HPLC: 86.7%;
[0066] Add the above-mentioned 3-hydroxyphthalic acid (24.1 g, HPLC: 86.7%) in the reaction flask, add acetic anhydride (27.04 g, 482 mmol), stir and heat to 70 ° C for 2 hours, after the reaction, the reaction solution is depressurized Concentrate to obtain 16.1 g of yellow oily liquid 3-hydroxyphthalic anhydride, with a yield of 70.7%.
[0067] H Spectrum: 1 H NMR(500MHz...
Embodiment 3
[0084] a. Preparation of 3-hydroxyphthalic anhydride
[0085] Add 3-chlorophthalic anhydride (22 grams, 120.5mmol), N,N-dimethylformamide (60mL), water (30mL) and catalyst cuprous bromide (4.18 grams, 24mmol) in the reaction flask, stirring , add potassium hydroxide (27.04 g, 482 mmol), and react at 110°C for 12 hours. After the reaction, filter with suction, wash the filter cake with dichloromethane, adjust the pH value of the filtrate to 1~2, extract with dichloromethane, and collect the organic solvent And concentrated to obtain 22.01 grams of reddish-brown oily liquid 3-hydroxyphthalic acid, HPLC: 81.9%;
[0086] Add the above-mentioned 3-hydroxyphthalic acid (22.01 grams, HPLC: 81.9%) in the reaction flask, add acetic anhydride (36.8 grams, 361.5 mmol), stir and heat to 80 ° C for 2 hours, after the reaction, the reaction solution decreases Concentrate under reduced pressure to obtain 15.1 g of reddish-brown oily liquid 3-hydroxyphthalic anhydride, with a yield of 62.9%....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com