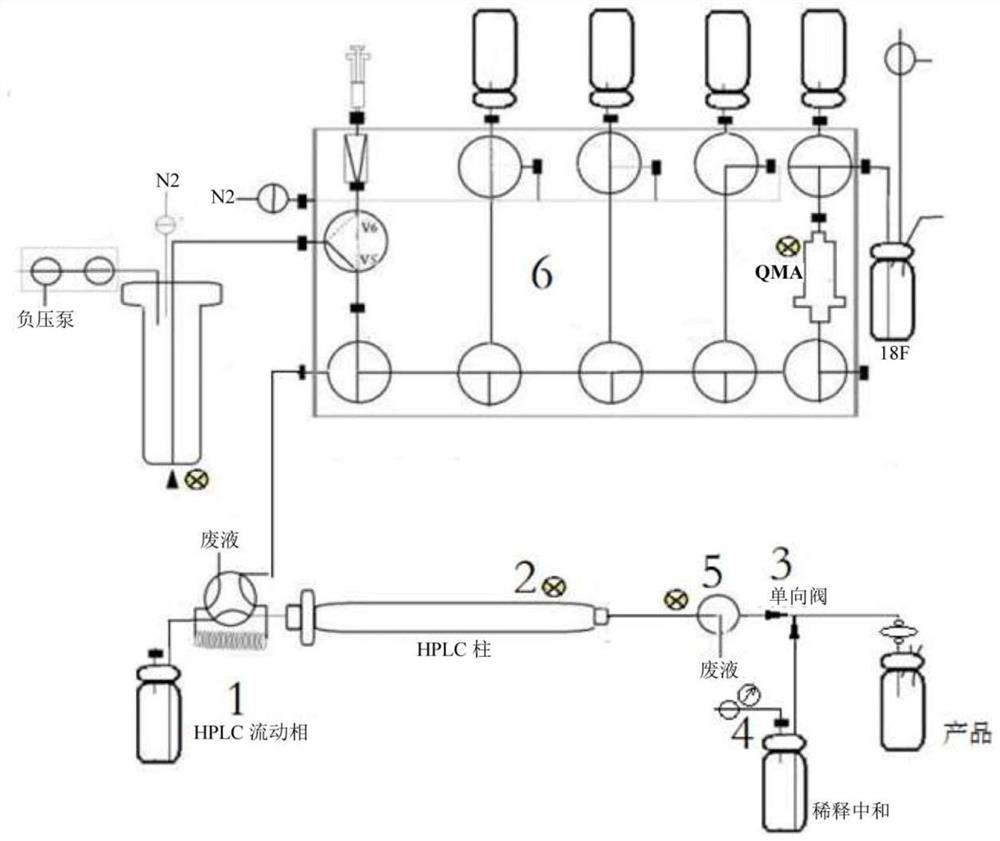
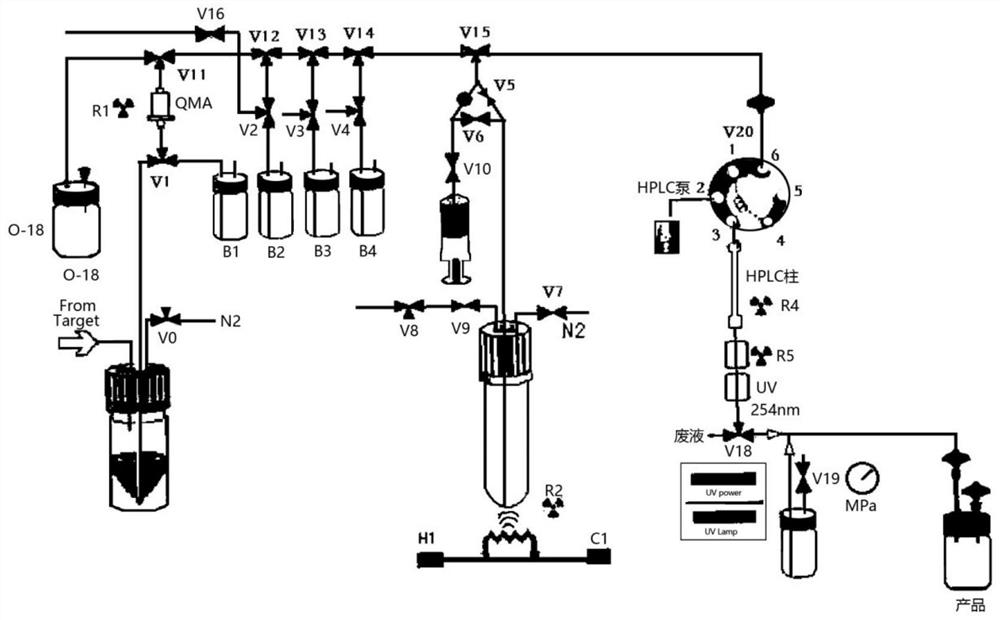
Clinical single-tube fluorine-18 multifunctional module equipment and radiopharmaceutical synthesis process
A multifunctional, clinical technology, applied in chemical/physical processes, chemical/physical/physical chemical processes, chemical/physical/physical chemical stationary reactors, etc. problems, to reduce the additional radiation dose, eliminate the interference of impurities, and avoid solvent conversion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 118
[0048] Example 1. 18 Synthesis of F-DCFPyL
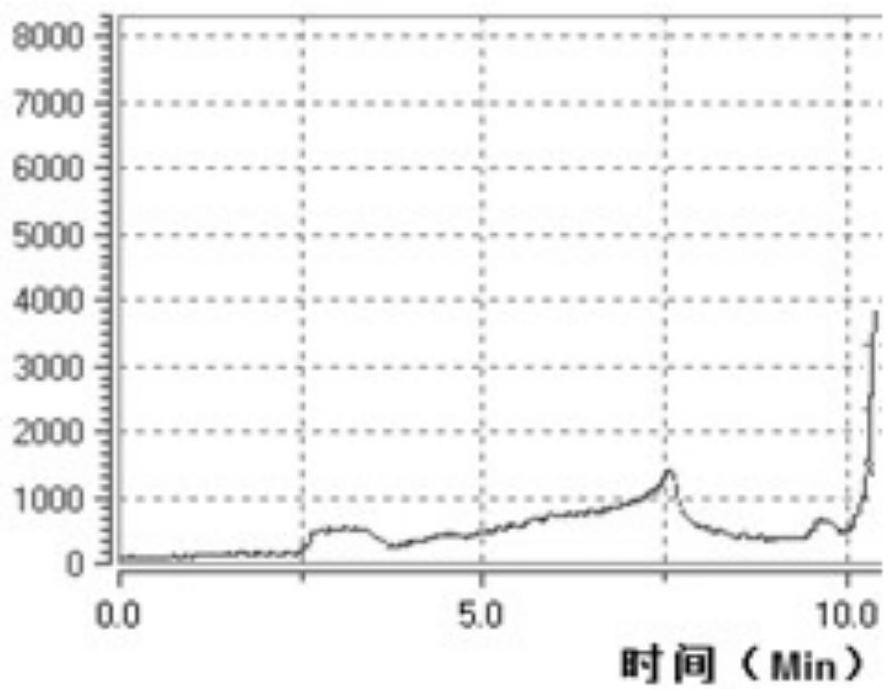
[0049] like image 3 Reagents were individually installed in the following vials and mounted on the module prior to synthesis as shown:
[0050] B1: 1 mL of TBA-containing acetonitrile solution; B2: 2 mL of acetonitrile; B3: 5 mg of precursor dissolved in 0.5 mL of acetonitrile; B4: 0.5 mL of 50% phosphoric acid (V / V); B5: 7 mL of HPLC mobile phase; B6: 10 mL of 0.5 mol / L NaHCO 3 .
[0051] The 2200mCi 18 The F ions are transferred from the accelerator to the QMA column, start the automatic program, and run automatically under the control of the computer: rinse the QMA column with the acetonitrile solution containing TBA in the B1 bottle into the reaction tube, heat, aerate and azeotropically remove water with acetonitrile, and then Add acetonitrile in bottle B2 to remove water repeatedly, cool to 45℃, add the precursor in bottle B3, nucleophilic reaction at 50℃ for 5min, then add phosphoric acid in bottle B4, hydrolyze at 50℃...
Embodiment 218F-7
[0054] Example 2. 18 Synthesis of F-7Q-PSMA
[0055] like image 3 As shown, the reagents were separately installed in the following vials prior to synthesis:
[0056] B1: 1 mL of acetonitrile solution containing TBA; B2: 2 mL of acetonitrile; B3: 0.5 mg of precursor dissolved in 0.5 mL of DMF; B4: empty; B5: 7 mL of HPLC mobile phase; B6: 10 mL of 0.5 mol / L NaHCO 3 .
[0057] The 800mCi 18 The F ions are transferred from the accelerator to the QMA column, start the automatic program, and run automatically under the control of the computer: rinse the QMA column with the acetonitrile solution containing TBA in the B1 bottle into the reaction tube, heat, aerate and azeotropically remove water with acetonitrile, and then Add the acetonitrile solution in bottle B2 to repeat water removal, cool to 45 °C, add the precursor in bottle B3, and conduct nucleophilic reaction at 50 °C for 5 min. After adding the HPLC mobile phase in bottle B5, separate the upper half of the mixture by...
Embodiment 318
[0060] Example 3. 18 Synthesis of F-FMSIO
[0061] like image 3 As shown, the reagents were separately installed in the following vials prior to synthesis:
[0062] B1: 1mL acetonitrile solution containing K2.2.2; B2: 2mL acetonitrile; B3: 5mg precursor dissolved in 1mL acetonitrile; B4: 2ml 1mol / L hydrochloric acid; B5: 1mL 2mol / L NaOH and 5mL HPLC mobile phase mixture; B6: empty .
[0063] The 1940mCi 18 The F ions are transferred from the accelerator to the QMA column, start the automatic program, and run automatically under computer control: rinse the QMA column with the acetonitrile solution containing K2.2.2 in the B1 bottle and put it into the reaction tube, heat, aerate and azeotropically remove water with acetonitrile, Then add the acetonitrile solution in the B2 bottle to repeat the water removal, cooling, add the precursor in the B3 bottle, nucleophilic reaction at 115 ℃ for 5 minutes, then add the hydrochloric acid solution in the B4 bottle, hydrolyze at 110 ℃...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com