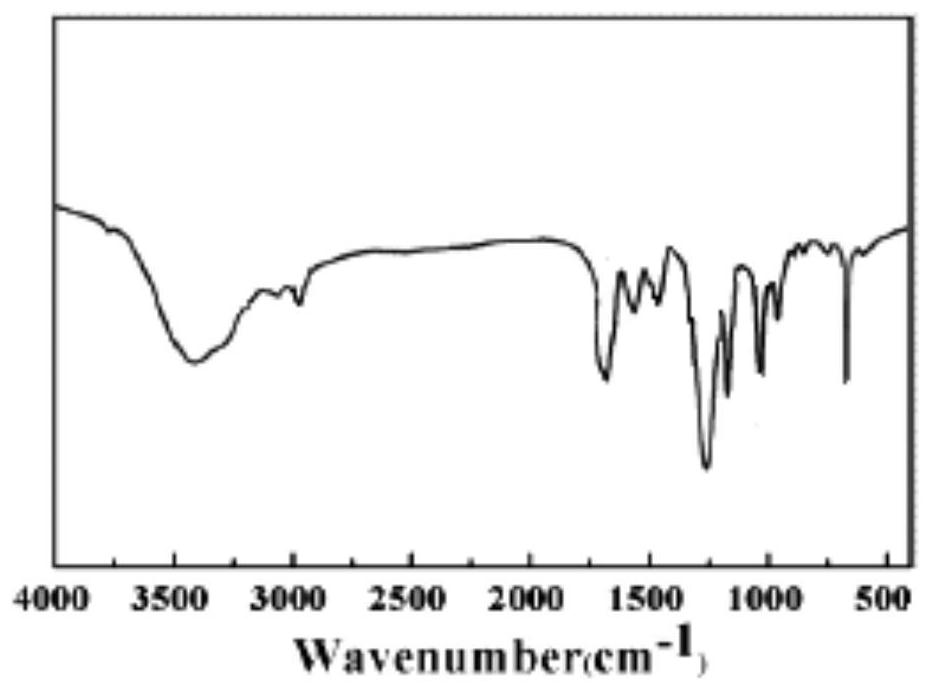
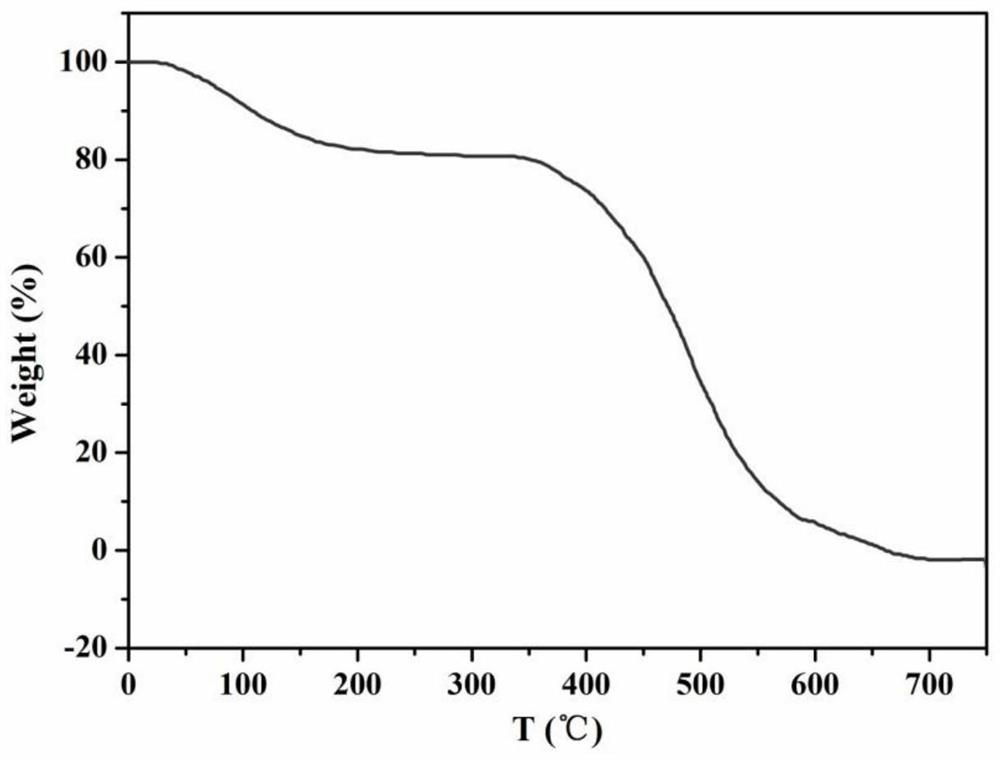
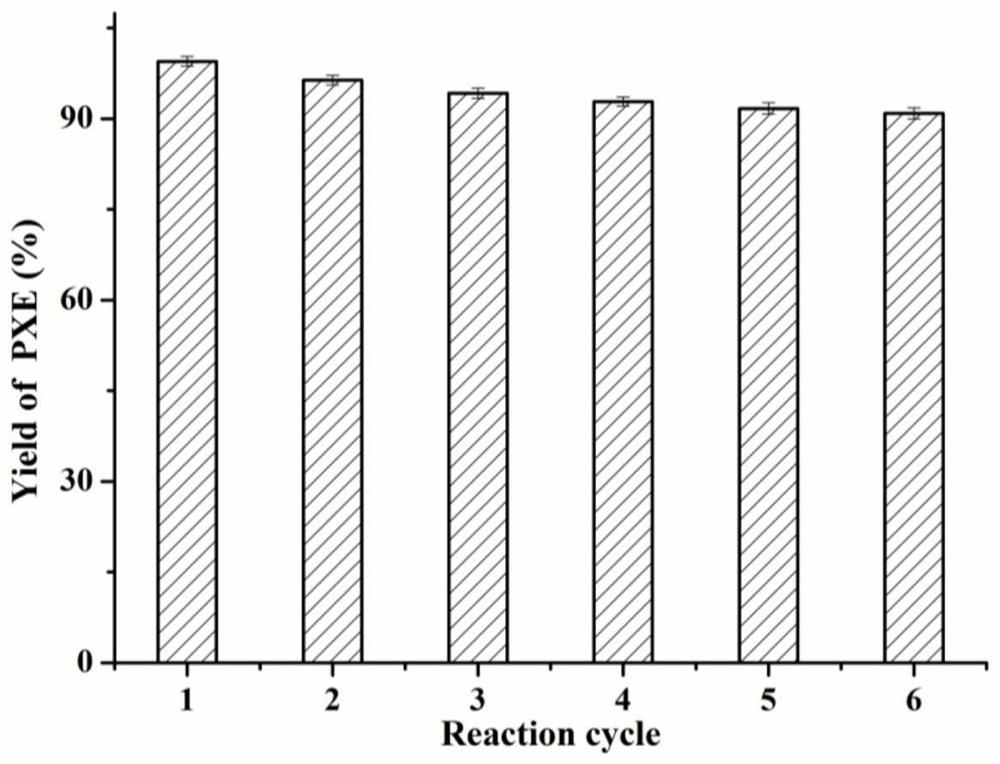
Preparation method of phenylacetic acid
A production method and technology of phenylacetic acid, applied in the preparation of carboxylate salts, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problem of low atom utilization in synthesis methods, increased reaction pressure conditions, equipment, and increased demand for high-pressure resistance, etc. problems, achieve good recycling performance, solvent recyclability, and reduce usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A kind of preparation of catalyst for the production process of phenylacetic acid, comprising:
[0034] S1: Weigh the monomer N-vinylimidazole and L-α-phosphatidylcholine-β-arachidonoyl-γ-stearoyl (the mass ratio of the two is 1: 2.47) and dissolve it in DMF (with the monomer The liquid-solid ratio of N-vinylimidazole is 44.1mL: 1g), add AIBN (the mass ratio of monomer N-vinylimidazole is 0.12:1), stir mechanically at room temperature for 3h, and transfer the mixture to 50mL of high-pressure In a reaction kettle, conduct a hydrothermal reaction at 100°C for 24 hours; vacuum-dry at 40°C until the solvent is completely evaporated, and grind the obtained solid into powder to obtain the product M;
[0035] S2: Dissolve the product M in ethanol (the solid-to-liquid ratio is 1g: 25ml), and add n-bromoheptane (the product M and its solid-to-liquid ratio is 1g: 2.5mL) in the dark, and centrifuge after 12h under magnetic stirring Wash with a large amount of ethanol to remove ex...
Embodiment 2
[0041] A kind of phenylacetic acid production process is identical with embodiment 1 with the preparation of catalyst.
[0042] The difference between the production of phenylacetic acid and Example 1 is that in the Friedel-Crafts alkylation reaction, the mass ratio of benzene, product A, and catalyst is 1:3.86:0.43.
Embodiment 3
[0044] A kind of phenylacetic acid production process is identical with embodiment 1 with the preparation of catalyst.
[0045]The difference between the production of phenylacetic acid and Example 1 is that in the Friedel-Crafts alkylation reaction, the mass ratio of benzene, product A, and catalyst is 1:3.8:0.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com