Synthesis method of DL-cysteine
A synthesis method and cysteine technology, applied in the field of DL-cysteine synthesis, can solve the problems of high toxicity and high price, and achieve the effects of reaction safety, short reaction steps and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
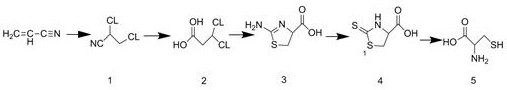
[0027] The synthetic method of embodiment 1 DL-cysteine, refer to figure 1 As shown, the details are as follows:
[0028] (1) Add 50g of acrylonitrile to a three-necked reaction flask, add catalyst magnesium carbonate, the amount of catalyst used is 10% of the weight of acrylonitrile, and slowly introduce 70g of chlorine gas below 0°C to maintain the reaction temperature between 12°C and chlorine gas After passing through, it was incubated at 12°C for 3 hours, then fed into nitrogen to drive off excess chlorine gas, and the reaction ended to obtain 114.7g of 2,3-dichloropropionitrile ( figure 1 The middle mark is 1), the yield is 98.1%, and the purity is 98.4%;
[0029] (2) Add a mixed medium of 85% acetic acid, concentrated sulfuric acid and water with a volume ratio of 1:1:1.2 to the reaction flask, then add 100 g of 2,3-dichloropropionitrile prepared in step (1), and then Reflux at 115°C for 1 hour to obtain 102.6 g of 2,3-dichloropropionic acid ( figure 1 The winning nu...
Embodiment 2
[0033] The synthetic method of embodiment 2 DL-cysteine, refer to figure 1 As shown, the details are as follows:
[0034] (1) Add 50g of acrylonitrile to a three-necked reaction flask, add catalyst magnesium carbonate, the amount of catalyst used is 12% of the weight of acrylonitrile, slowly introduce 70g of chlorine gas below 3°C, keep the reaction temperature between 17°C, chlorine gas After passing through, it was incubated at 17° C. for 4 hours, then fed into nitrogen to drive out excess chlorine, and the reaction ended to obtain 107 g of 2,3-dichloropropionitrile ( figure 1 The middle mark is 1), the yield is 92%, and the purity is 99%;
[0035] (2) Add a mixed medium of 85% acetic acid, concentrated sulfuric acid and water with a volume ratio of 1:1:1.2 to the reaction flask, then add 100 g of 2,3-dichloropropionitrile prepared in step (1), and then Reflux at 120°C for 1.2 hours to obtain 100.8 g of 2,3-dichloropropionic acid ( figure 1 The winning number is 2), the y...
Embodiment 3
[0039] The synthetic method of embodiment 3 DL-cysteine, refer to figure 1 As shown, the details are as follows:
[0040] (1) Add 50g of acrylonitrile to a three-necked reaction flask, add catalyst magnesium carbonate, the amount of catalyst used is 11% of the weight of acrylonitrile, slowly introduce 70g of chlorine gas below 0°C, and keep the reaction temperature between 14°C and chlorine gas After passing through, keep warm at 14 DEG C for 3 hours, then feed nitrogen to drive off excess chlorine, and the reaction ends to obtain 115g of 2,3-dichloropropionitrile ( figure 1 The middle mark is 1), the yield is 98.4%, and the purity is 98.9%;
[0041] (2) Add a mixed medium of 85% acetic acid, concentrated sulfuric acid and water with a volume ratio of 1:1:1.2 to the reaction flask, then add 100 g of 2,3-dichloropropionitrile prepared in step (1), and then Reflux at 118°C for 1.2 hours to obtain 104 g of 2,3-dichloropropionic acid ( figure 1 The middle mark is 2), the yield ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com