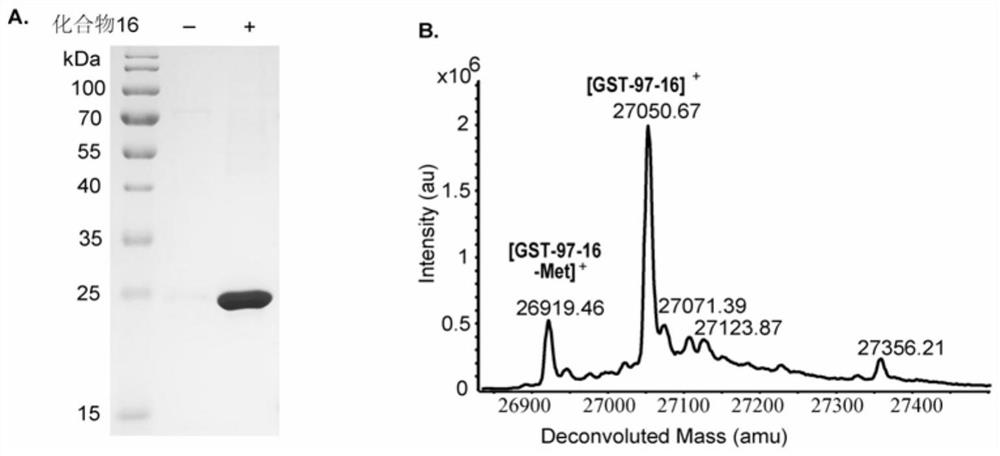
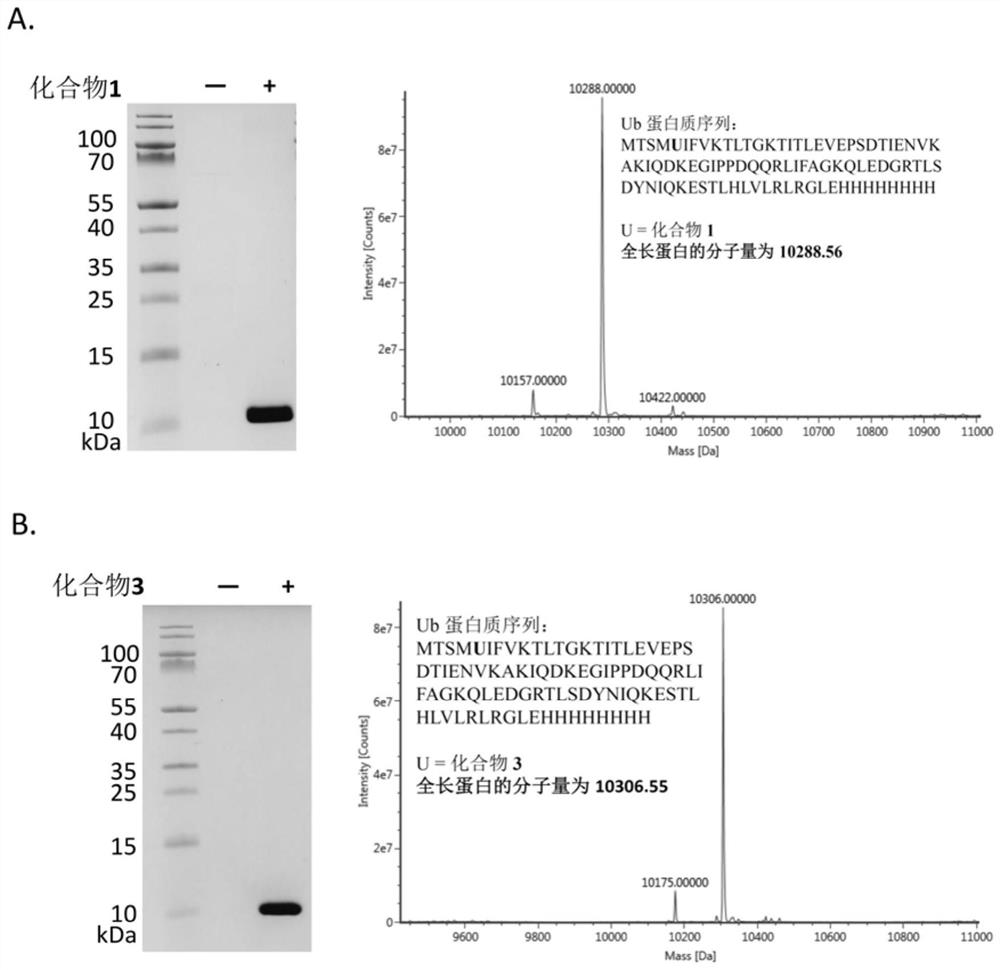
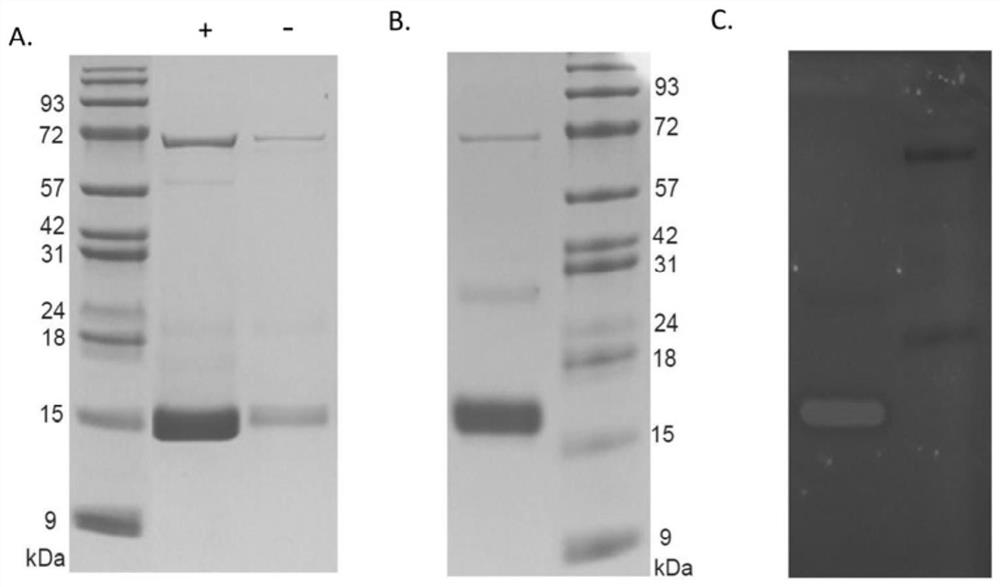
Non-natural amino acid and application thereof in protein site-specific modification and protein interaction
A protein and compound technology, applied in the field of protein-specific modification, can solve the problems of difficulty in identification of cross-linked proteins, few structural types, and low efficiency of unnatural amino acid expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117]
[0118] Step I-1: Dissolve 4-bromomethyl-3-nitrobenzoic acid (5.0 g, 19.31 mmol, 1.0 eq) in 60 mL of acetone, add 60 mL of water, and stir well. Anhydrous sodium carbonate (7.16 g, 67.58 mmol, 3.5 eq) was then added. The resulting mixture was reacted at 70°C for 2 hours. The reaction was monitored by LC-MS. After the reaction was completed, the pH was adjusted to 3-4 with 2N hydrochloric acid, extracted with ethyl acetate, dried, and the solvent was spun off to obtain 4.02 g of a yellow solid, which was directly injected into the next step without further purification. ESI-MS[M-H] - m / z=196.09. 1 H NMR (400MHz, CDCl 3 )δ8.79(d, J=1.6Hz, 1H), 8.36(dd, J=8.1, 1.7Hz, 1H), 7.96(d, J=8.2Hz, 1H), 5.11(s, 2H).
[0119] Step I-2: Dissolve the compound obtained in the previous step in 50 mL of anhydrous N,N-dimethylformamide, add tert-butyldimethylsilyl chloride (5.82 g, 38.62 mmol, 2.0 eq) and imidazole (2.63 g, 38.62 mmol, 2.0 eq). The resulting mixture was stirred ...
Embodiment 2
[0126]
[0127] Step II-1: At room temperature, add N-Boc-L-lysine (10.0g, 40.60mmol, 1.0eq) to a 500mL round bottom flask containing 100mL of tert-butanol solution, add di-tert-butyl dicarbonate (6.08g, 27.86mmol, 1.06eq), then added 4-dimethylaminopyridine ((1.61g, 13.14mmol, 0.5eq), and the resulting mixture was stirred at room temperature overnight. After the reaction was completed, the t- butanol, the residual solid was redissolved in 300mL of ethyl acetate, and then washed successively with saturated ammonium chloride and saturated aqueous sodium chloride.The organic phase was dried over anhydrous sodium sulfate and concentrated in vacuo to obtain 10.26g of a colorless oily crude The product. The oil was directly used in the next reaction without purification.
[0128] Step II-2: Dissolve the crude product from the previous step in 200 mL methanol solution at room temperature, add ammonium formate (7.58 g, 120.25 mmol, 5.0 eq), and then add Pd-C (10%, 1.00 g). The re...
Embodiment 3
[0132]
[0133] Step III-1: Compound III-A (5.0g, 23.04mmol, 1.0eq) was dissolved in dry tetrahydrofuran, and borane (69.12mL, 3.0eq) was added under ice-cooling conditions, and heated to 50°C for 2 hours. After the reaction was completed, methanol was added to quench the reaction, the solvent was swirled away, and purified by column chromatography to obtain 4.42 g of a brown product with a yield of 95%. 1 H NMR (400MHz, DMSO) δ9.85(s, 1H), 7.22(d, J=8.1Hz, 1H), 6.96(dd, J=8.1, 2.0Hz, 1H), 6.92(d, J=1.9Hz ,1H),5.03(s,1H),4.41(s,2H).
[0134] Step III-2: Dissolve compound III-B (4.08g, 20.09mmol, 1.0eq) in acetone, add anhydrous sodium sulfate (14.27g, 100.45mmol, 5.0eq), p-toluenesulfonic acid (380mg, 2.0mmol , 0.1eq), and 2,2-dimethoxypropane (21.0g, 200.9mmol, 10.0eq), reacted at 40°C. After the reaction was completed, it was diluted with ethyl acetate, washed with saturated sodium bicarbonate and saturated sodium chloride successively, dried, and passed through the col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com