Ampelopsin oral preparation with high bioavailability
A technology for oral preparations of staphylosin, which is applied in the field of improving or enhancing the bioavailability of oral absorption of staphylosin, which can solve problems such as differences, bioavailability that has not been further studied and verified, and irregularity, etc., to achieve simple process, Effect of promoting dissolution and absorption and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Effects of different excipients on the metabolism of staphylophyllin in rat liver microsomes
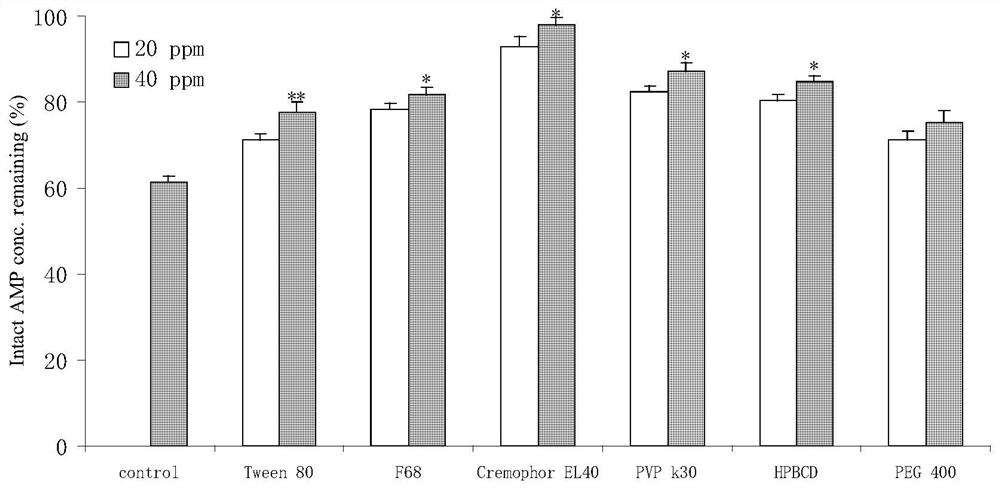
[0026] Select 6 kinds of excipients (polyoxyethylene hydrogenated castor oil 40, Tween-80, hydroxypropyl-β-cyclodextrin, Pluronic F68, povidone K30, PEG400), and add them to the liver of rats in a certain amount. In the microbody (protein concentration 1.0 mg / ml) incubation system, shake at 100 rpm for 15 minutes in a water bath at 37°C, add staphylophyllin (80 μmol / l) and NADPH coenzyme (1 mmol / l) to start the reaction, and after 10 minutes, add three Double the amount of ice methanol to terminate the reaction, vortex for 1 min, and centrifuge in a low-temperature high-speed centrifuge (4°C, 13,000 rpm) for 8 min, take the supernatant, and compare the remaining staphylophyll by LC-MS / MS.
[0027] The result is as figure 1 As shown, low and high doses (20, 40 μg / ml) of polyoxyethylene hydrogenated castor oil RH40, Tween-80, hydroxypropyl-β-cyclodextrin, pluronic F68...
Embodiment 2
[0028] Example 2 Determination of the IC50 value of five kinds of excipients on the inhibition of staphyloclin metabolism
[0029]Five kinds of excipients were used in different concentration ranges: polyoxyethylene hydrogenated castor oil 40 (1.0-40.00 μg / ml), Tween-80 (10.0-200.0 μg / ml), hydroxypropyl-β-cyclodextrin ( 4.0-100.0μg / ml), Pluronic F68 (10.0-200.0μg / ml), povidone K30 (4.0-100.0μg / ml), were added to rat liver microsomes (protein concentration 1.0 mg / ml) incubation system, after shaking at 100rpm in a 37°C water bath for 15min, add staphylophyllin (80μmol / l) and NADPH coenzyme (1mmol / l) to start the reaction, and after 10min, add three times the amount of ice methanol to terminate the reaction. Vortex for 1 min, then centrifuge in a low-temperature high-speed centrifuge (4°C, 13,000 rpm) for 8 min, take the supernatant, and compare the remaining amount of staphylophyll by LC-MS / MS, and calculate the IC50 value of each excipient (see Table 1). The results showed t...
Embodiment 3
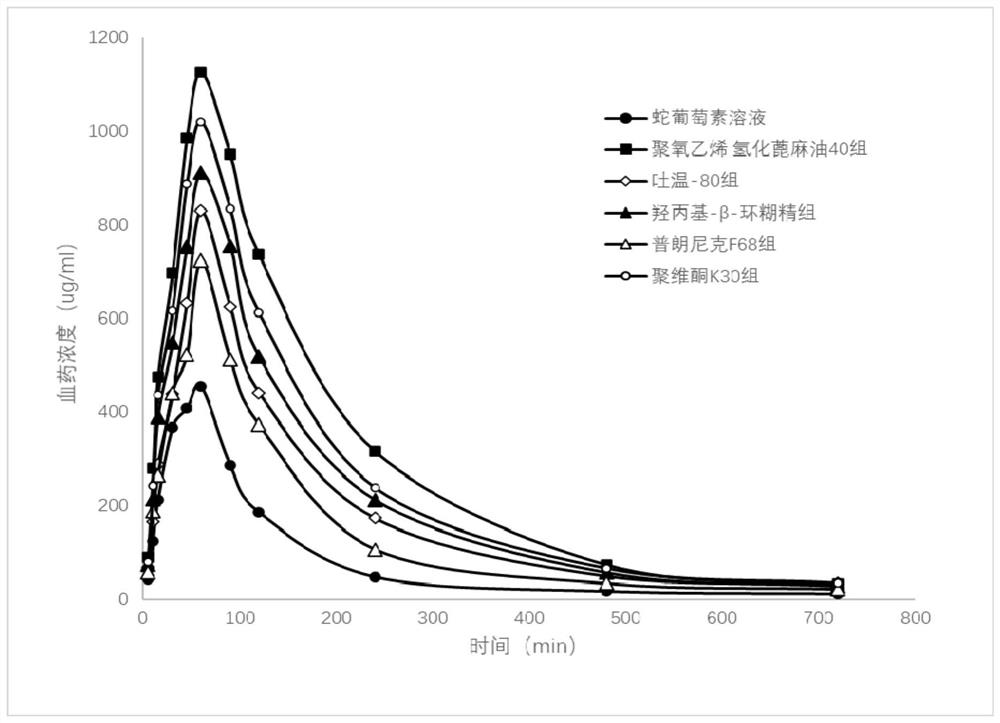
[0032] Embodiment 3 The pharmacokinetics comparison of adjuvant to the oral administration of staphylocoxine
[0033] Mix a certain proportion of metabolic inhibitory excipients (polyoxyethylene hydrogenated castor oil 40, Tween-80, hydroxypropyl-β-cyclodextrin, pluronic F68, povidone K30) with a certain concentration of staphylophyllin Mix to make a solution. Taking prednisin alone as a control, the rats were administered by intragastric administration, blood was collected from the orbit, and the concentration of the drug in plasma was measured at each time point. The operation is as follows:
[0034] Rats (male, body weight 180-220 g) were randomly divided into 6 groups, 5 rats in each group. Fasted overnight before administration and had free access to water. The mixed solution of adjuvant and staphylophyll of the same dosage was given by intragastric administration respectively, and a separate saponin solution (the hydrotrope was dissolved in the aqueous solution contai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com