Botulinum toxin cell binding domain polypeptides and methods of use for treatments of fibrosis associated disorders
A botulinum toxin, binding domain technology, applied in fusion polypeptides, chemical instruments and methods, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
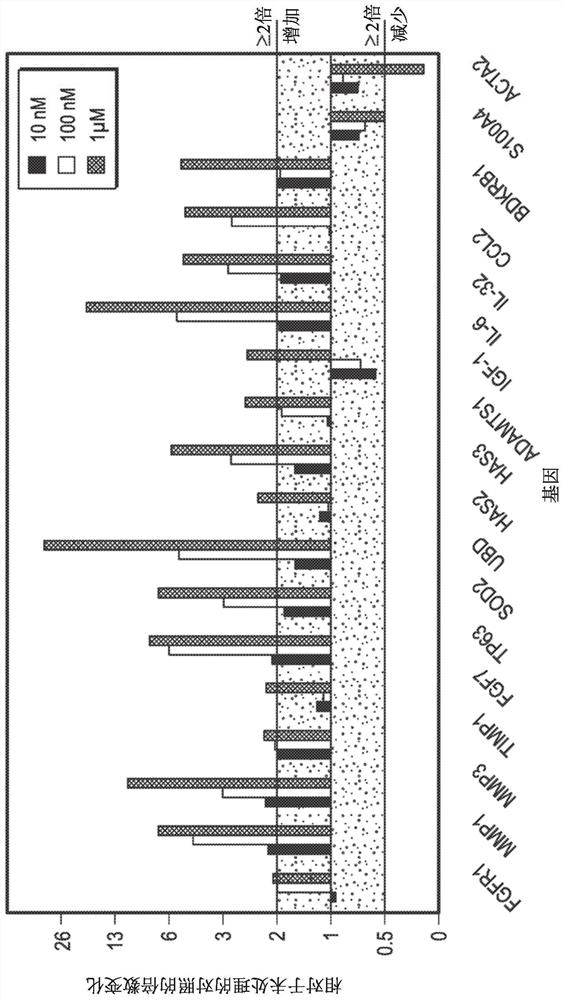
[0346] Effects of the exemplary polypeptide of SEQ ID NO: 19 on cellular gene expression in normal human primary fibroblasts
[0347] With 10nM, 100nM or 1μM with the binding domain of BoNT / A (H C / A) Treat normal human primary fibroblasts with polypeptides having substantially the same amino acid sequence for 1 day (24 hours), resulting in significant dose-dependent changes in the expression of 16 fibroblast genes based on qPCR. Briefly, adult human dermal fibroblasts (HDFa) (ThermoFisher Scientific, catalog number C0135C) were cultured in MEM medium containing 2% FBS for 2 weeks, and then treated with 10 nM, 100 nM or 1 μM of SEQ ID NO: 19 peptides were treated for 1 day (24 hours). Expression of genes known to be expressed in fibroblasts and involved in ECM organization, inflammation or epidermal self-renewal (keratinocyte stem cell factor) was evaluated. cDNA was generated by reverse transcription using the SUPERSCRIPT VILO cDNA Synthesis Kit (Thermo Scientific #11754050),...
Embodiment 2
[0349] Effect of the exemplary polypeptide of SEQ ID NO: 21 on cellular gene expression in pimple scar-derived human primary fibroblasts
[0350] With the N-terminal half (H CN Treatment of pimple scar primary human fibroblasts with a polypeptide (SEQ ID NO: 21) having substantially the same amino acid sequence as / A) produced significant dose-dependent changes in the expression of six (6) fibroblast genes based on qPCR . Briefly, human dermal fibroblasts (KF116R) (cellResearchCorp Pte Ltd) were cultured in MEM medium containing 2% FBS for 2 weeks, and then treated with 1 nM, 10 nM or 1 μM of the polypeptide of SEQ ID NO: 211 day (24 hours). The expression of six (6) fibroblast-associated genes known to be involved in ECM organization and remodeling (MMP1, MMP3, and TIMP1), and epidermal differentiation and self-renewal (FGFR1, FGF7, and TP63) were evaluated. cDNA was generated by reverse transcription using the SUPERSCRIPT VILO cDNA Synthesis Kit (Thermo Scientific #117540...
Embodiment 3
[0352] Effect of the exemplary polypeptide of SEQ ID NO: 19 on collagen secretion from human primary fibroblasts derived from pimple scars
[0353] With 1μM with the binding domain of BoNT / A (H C / A) Polypeptide (SEQ ID NO: 19) with substantially the same amino acid sequence treats pimple scar human primary fibroblasts, so that the type I precursor secreted by pimple scar human primary fibroblasts into a medium containing 10% FBS Collagen C-peptide (PIP) was significantly reduced by about 30%. This reduction was similar to that observed after treatment with 0.1 μM antibody to TGF-β (approximately 20%) or 1 μM antibody to CTGF (approximately 30%). Briefly, human dermal pimple fibroblasts (KF116R) were regularly cultured in MEM medium containing 2% FBS. For experiments, cells were kept in 2% FBS medium or switched to medium containing 10% FBS with or without 1 μM of the polypeptide of SEQ ID NO: 19, 0.1 μM of anti-TGF-β mAb or 1 μM of anti-CTGF mAb deal with. After 24 hours,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com