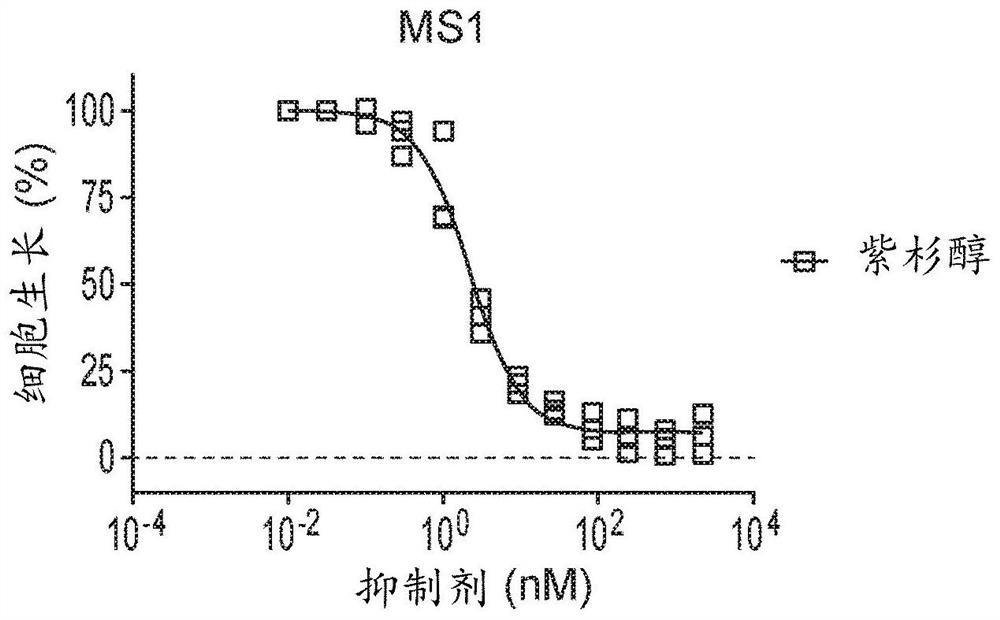
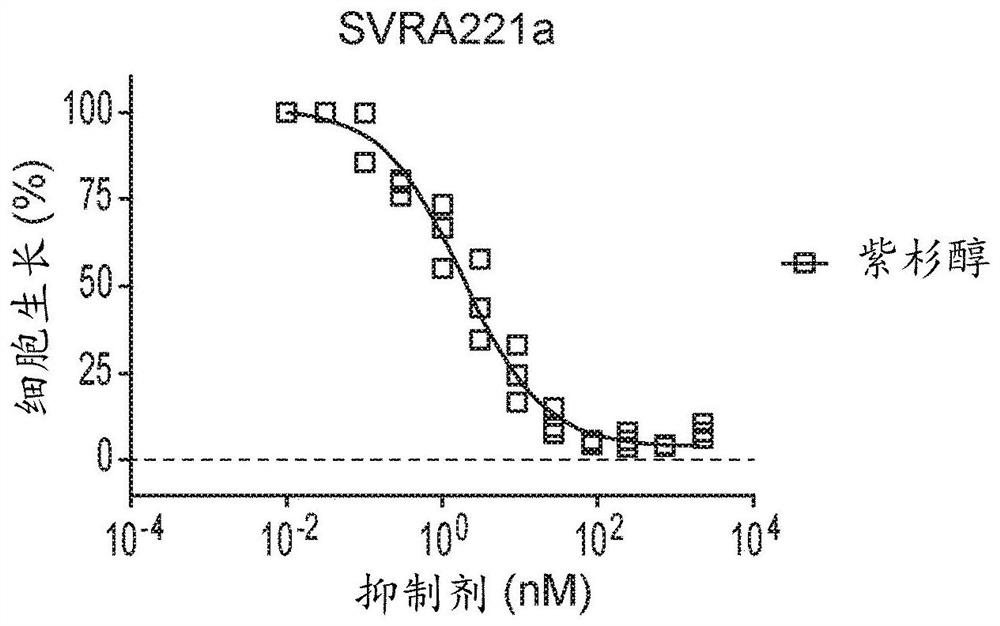
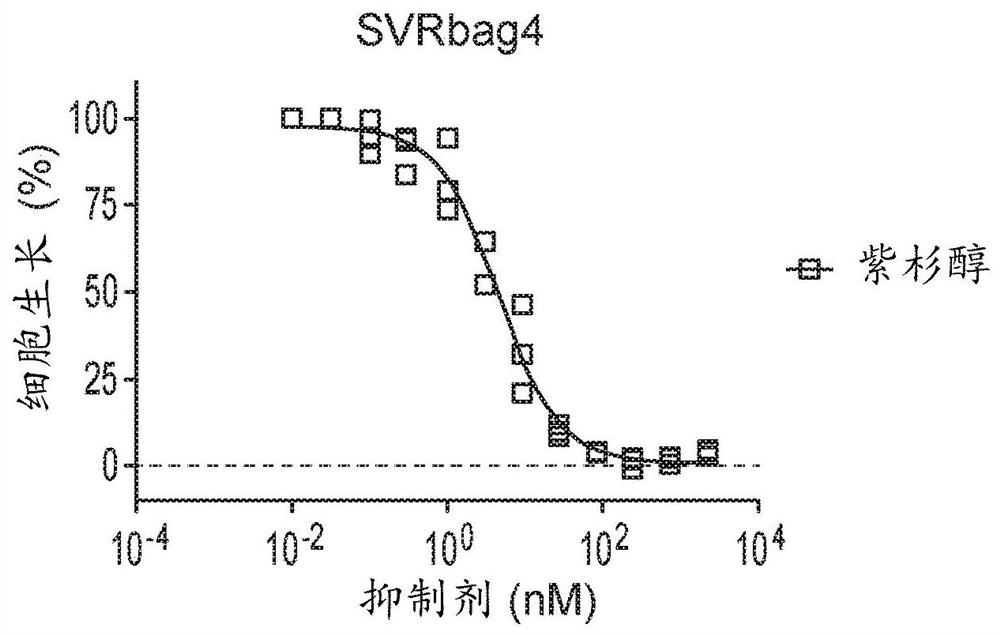
Therapeutic combinations of orally administered paclitaxel and a p-gp inhibitor for the treatment of angiosarcoma
A technology of angiosarcoma and paclitaxel, which is applied in the field of compounds for the treatment of angiosarcoma, and can solve problems such as inability to inject paclitaxel intravenously and reluctance to accept it
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[3574] Example 1 - Combination of Orally Administered Paclitaxel and Compound A in Vivo in a Tumor Cell Implanted Mouse Model anticancer activity
[3575] The ability of orally administered paclitaxel in combination with Compound A to inhibit the growth of different human tumor xenograft cell lines was investigated in 7 in vivo experiments in mice. Paclitaxel administered intravenously (IV) or intraperitoneally (IP) alone served as a control. A summary and results of the study are provided in Table 1 below.
[3576] Table 1: Summary and results of the in vivo anticancer activity of orally administered Paclitaxel in combination with Compound A in a tumor cell implanted mouse model
[3577]
[3578]
[3579] In these studies, tumor inhibition rate (IR%) was calculated as ([1 - relative tumor growth in treatment group / relative tumor growth in control group] x 100). Tumor regression was calculated as (mean tumor weight 治疗结束 / average tumor weight 治疗开始 )x100. Thus, a ...
Embodiment 2
[3582] Example 2 - Dose-Dependence of Orally Administered Paclitaxel
[3583] In rats and dogs orally administered paclitaxel in combination with Compound A, paclitaxel C max and AUC increased in a greater than dose proportional manner, as shown in Tables 2 and 3 below.
[3584] Table 2: Dose dependence of paclitaxel in rat pharmacokinetics after single oral administration in combination with compound A
[3585]
[3586] Table 3: Dose Dependence of Paclitaxel in Dog Pharmacokinetics Following Single-Dose Oral Administration in Combination with Compound A
[3587]
Embodiment 3
[3588] Example 3 - Determination of the Bioavailability of Orally Administered Paclitaxel in Combination with Compound A in Subjects with Cancer Utilization rate
[3589] The study was designed to determine the absolute bioavailability of orally administered paclitaxel in combination with Compound A in subjects with cancer. Eligible subjects were instructed to take 80mg / m2 once a week over 1 hour 2 Dosage of intravenous paclitaxel (eg, or use Adults treated with paclitaxel formulated together.
[3590] The first 6 subjects received the following treatment approximately 1 week apart: 80 mg / m 2 Intravenous paclitaxel (as or generic drug) via 1 hour infusion (on day 1 or day 8) plus premedication according to standard local practice; or 15mg Compound A + 270mg (approximately 150mg / m 2 ) paclitaxel administered orally (days 1 and 2 or days 8 and 9). Compound A was administered 1 hour before oral administration of paclitaxel. Premedication is not permitted during this...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com