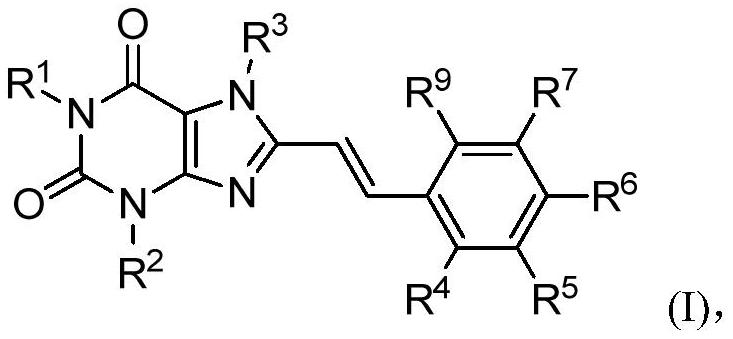
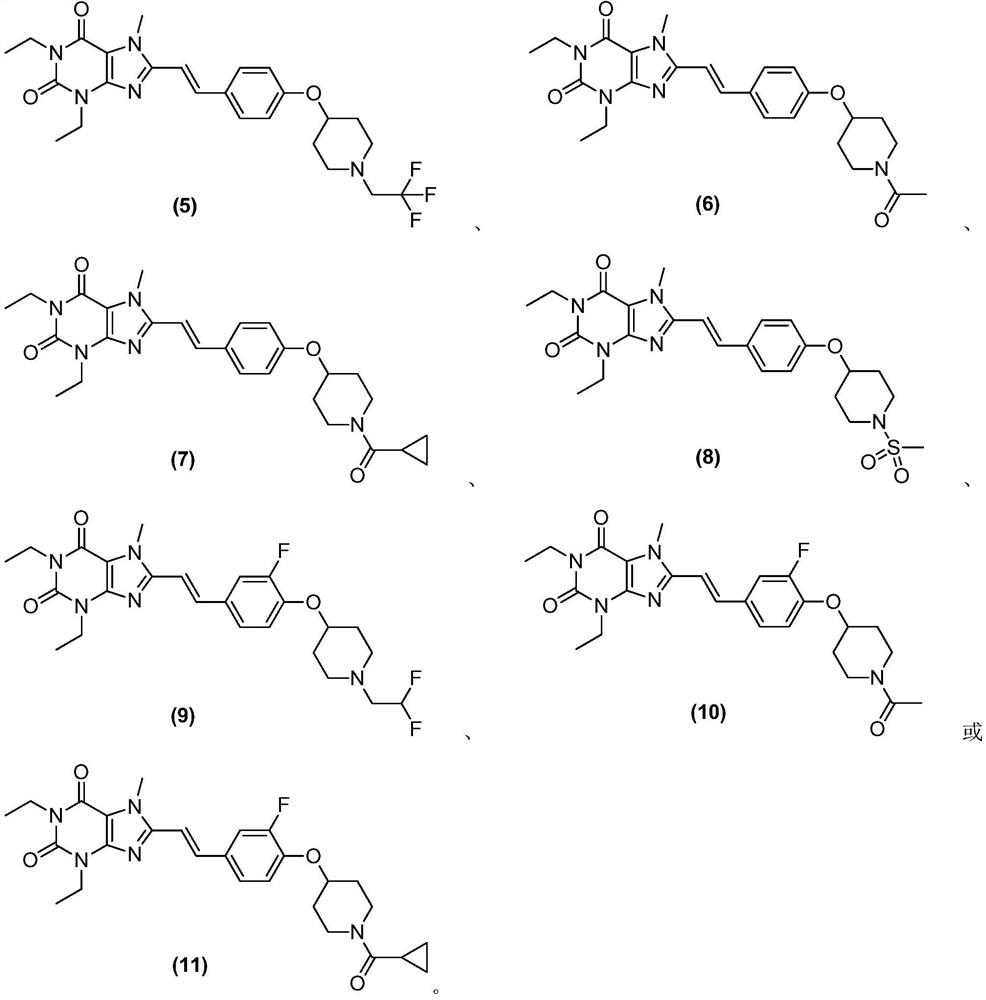
8-substituted styryl xanthine derivative and application thereof
An alkyl and aryl technology, applied in drug combinations, active ingredients of heterocyclic compounds, blood diseases, etc., can solve problems such as adenosine A distribution limitation, achieve good brain/plasma ratio, good pharmacokinetic properties, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
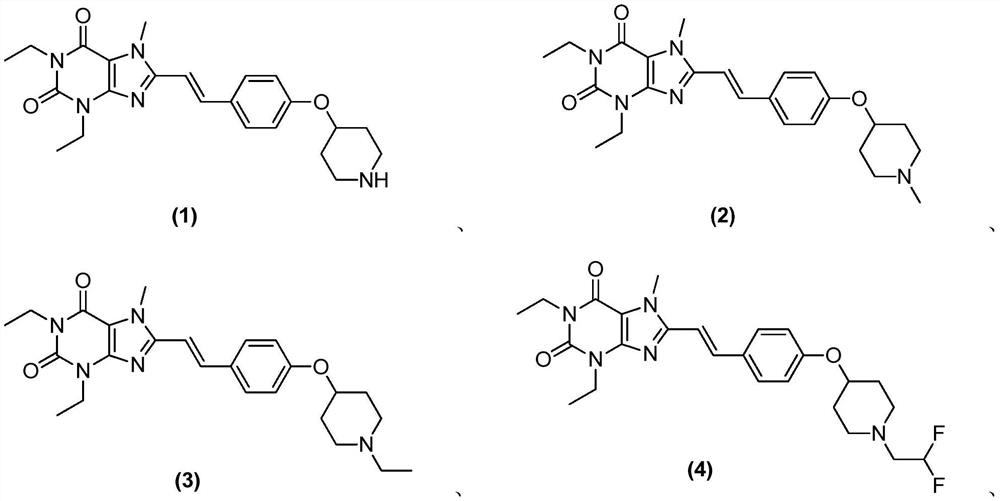
[0269] Example 1 (E)-1,3-diethyl-7-methyl-8-(4-(piperidin-4-yloxy)styryl)-1H-purine-2,6(3H, Synthesis of 7H)-diketones
[0270]
[0271] Step 1) Synthesis of tert-butyl 4-(p-toluenesulfonyloxy)piperidine-1-carboxylate
[0272]
[0273] At 25°C, p-toluenesulfonyl chloride (7.1g, 37.0mmol) and tert-butyl 4-hydroxypiperidine-1-carboxylate (5.0g, 24.8mmol) were added to a 100mL single-necked flask, and dichloromethane ( 40mL), then triethylamine (10.4mL, 74.2mmol) was added dropwise, and the reaction was continued for 18 hours; saturated sodium bicarbonate solution (30mL) was added, the liquid was separated, the organic phase was collected, spin-dried under reduced pressure, and separated and purified by column chromatography (Petroleum ether / ethyl acetate (v / v)=10 / 1~5 / 1) the title compound was obtained as a white solid (5.6 g, 63%).
[0274] MS(ESI,pos.ion)m / z:300.0[M+H-56] + ;
[0275] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.82 (d, J = 8.2Hz, 2H), 7.36 (d, J = 8.1Hz, 2H...
Embodiment 2
[0305] Example 2 (E)-1,3-diethyl-7-methyl-8-(4-((1-methylpiperidin-4-yl)oxy)styryl)-1H-purine- Synthesis of 2,6(3H,7H)-diketones
[0306]
[0307] (E)-1,3-diethyl-7-methyl-8-(4-(piperidin-4-yloxy)styryl)-1H-purine-2,6( 3H,7H)-diketone (0.5g, 1.18mmol) and methanol (10mL) were added to a 100mL single-necked round-bottomed flask, and aqueous formaldehyde (0.88mL, 12mmol, 37mass%) and acetic acid (142mg, 2.36mmol) were added, stirred After reacting for 15 minutes, add sodium cyanoborohydride (227mg, 3.54mmol), transfer to 25°C and continue stirring for 12 hours; stop the reaction, add water (30mL), dichloromethane extraction (50mL), liquid separation, organic phase After spin-drying under reduced pressure, separation and purification by column chromatography (dichloromethane / methanol (v / v)=15 / 1) gave the title compound as an off-white solid (0.49 g, 94.8%). MS(ESI,pos.ion)m / z:438.1[M+H] + ;
[0308] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.75 (d, J = 15.7Hz, 1H), 7.54 (d, J = ...
Embodiment 3
[0309] Example 3 (E)-1,3-diethyl-7-methyl-8-(4-((1-ethylpiperidin-4-yl)oxy)styryl)-1H-purine- Synthesis of 2,6(3H,7H)-diketones
[0310]
[0311] (E)-1,3-diethyl-7-methyl-8-(4-(piperidin-4-yloxy)styryl)-1H-purine-2,6( 3H,7H)-diketone (500mg, 1.18mmol) and acetonitrile (5mL) were added to a 50mL single-necked round bottom flask, N,N-diisopropylethylamine (0.56mL, 3.39mmol) was added, and then iodine was added dropwise Ethane (0.191mL, 2.38mmol), continue stirring for 3 hours; stop the reaction, spin dry under reduced pressure, separate and purify by column chromatography (dichloromethane / methanol (v / v)=30 / 1) to obtain the title compound as light Yellow solid (0.455 g, 85.3%).
[0312] MS(ESI,pos.ion)m / z:452.2[M+H] + ;
[0313] 1 H NMR (400MHz, CDCl 3 )δ7.75(d, J=15.7Hz, 1H), 7.57(d, J=8.6Hz, 2H), 6.95(d, J=8.6Hz, 2H), 6.81(d, J=15.7Hz, 1H) ,4.81(brs,1H),4.22(q,J=6.9Hz,2H),4.15–4.03(m,5H),3.47(brs,2H),3.30–3.08(m,4H),2.77(brs,2H ),2.34–2.18(m,2H),1.57(t,J=7.3Hz,3H),1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com