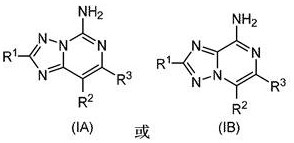
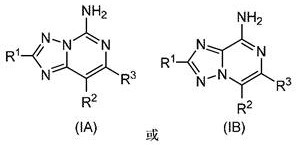
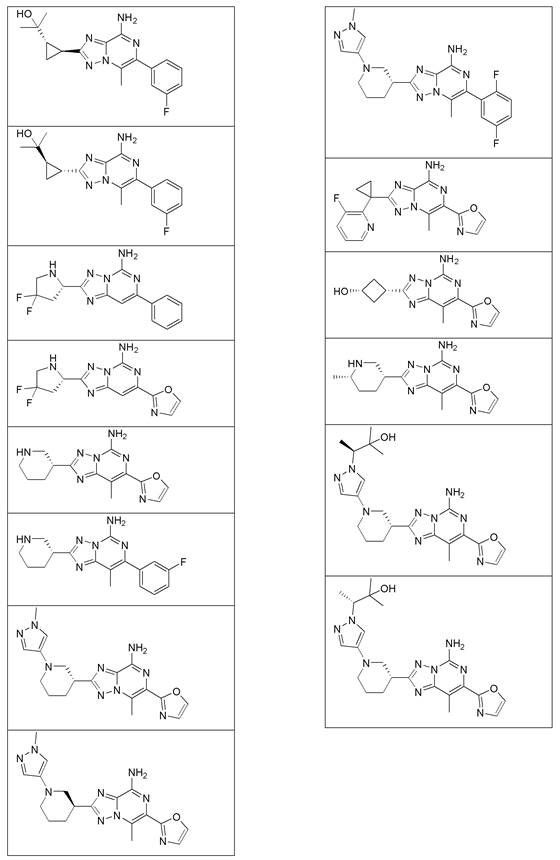
Substituted amino triazolopyrimidine and amino triazolopyrazine adenosine receptor antagonists, pharmaceutical compositions and their use
A compound, unsubstituted technology, applied in the direction of drug combination, antineoplastic drugs, medical preparations containing active ingredients, etc., can solve the problem of tumor immunotherapy enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
[0462] Example 1.2, 2-((1S,2S and 1R,2R)-2-(8-bromo-6-(3-fluorophenyl)-5-methyl-[1,2,4]triazole And[1,5-a]pyrazin-2-yl)cyclopropyl)propan-2-ol preparation of
[0463] plan 1
[0464]
[0465] Step 1 - Intermediate 1.1, 2-((1 S ,2 S and 1 R ,2 R )-2-(8-bromo-6-(3-fluorophenyl)-5-methyl-[1,2,4]triazolo[1,5 -a Synthesis of ]pyrazin-2-yl)cyclopropyl)propan-2-ol
[0466] Intermediate M.2 (22 mg, 0.052 mmol) and THF (525 µL) were charged in a 20 mL scintillation vial. The resulting mixture was cooled to -30 °C, then methylmagnesium bromide (3 M in Et 2 O, 44 µL, 0.131 mmol). The reaction mixture was warmed to 25 °C over 15 min and stirred for another 30 min. Then with saturated NH 4 Aqueous Cl solution (1 mL) quenched the reaction. DCM (1 mL) was added and the biphasic mixture was stirred for 5 min. The mixture was then diluted with water (2 mL) and extracted with DCM (3 x 4 mL). The combined organic layers were washed with anhydrous NaSO 4 Dried, filtered and co...
Embodiment 21
[0469] Example 2.1, (S)-2-(4,4-Difluoropyrrolidin-2-yl)-7-phenyl-[1,2,4]triazolo[1,5-c]pyrimidine Pyridine-5-amine, TFA salt preparation of
[0470] Scenario 2
[0471]
[0472] Will( S )-2-(5-((2,4-dimethoxybenzyl)amino)-7-phenyl-[1,2,4]triazolo[1,5 -c A mixture of tert-butyl]pyrimidin-2-yl)-4,4-difluoropyrrolidine-1-carboxylate (65 mg, 0.117 mmol) and TFA (1 mL) was heated at 60 °C for 1 h. Upon completion, the reaction was concentrated. The resulting crude residue was dissolved in DMSO (3 mL), filtered, and purified by reverse phase HPLC [Method A] to provide ( S )-2-(4,4-difluoropyrrolidin-2-yl)-7-phenyl-[1,2,4]triazolo[1,5 -c ] Pyrimidin-5-amine, TFA salt (Example 2.1). MS (ESI) m / z C 15 h 15 f 2 N 6 [M+H] + The calculated value is 317,1, and the measured value is 317.0. 1 H NMR (500 MHz, MeOD- d 4 ) δ 8.12-8.13 (m, 2 H), 7.46–7.50 (m, 4 H),5.42 (t, J = 8.5 Hz, 1 H), 3.95-4.91 (m, 2 H), 3.11-3.25 (m, 2 H). A2a IC 50 12.0 nM (A).
[0473] Table ...
Embodiment 2
[0480] Example 2.5-1 / 2.5-2
[0481] 5-methyl-2-(1-(1-methyl-1 H -pyrazol-4-yl)piperidin-3-yl)-6-(oxazol-2-yl)-[1,2,4]triazolo[1,5 -a ]pyrazin-8-amine by CHIRAL-Prep SFC [column: CC4, 21x250mm; 40% (0.1% NH 4 OH in MeOH) / CO 2 ; flow rate: 70 mL / min; 220 nm; the first elution peak (Example 2.5-1); the second elution peak (Example 2.5-2)] purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com