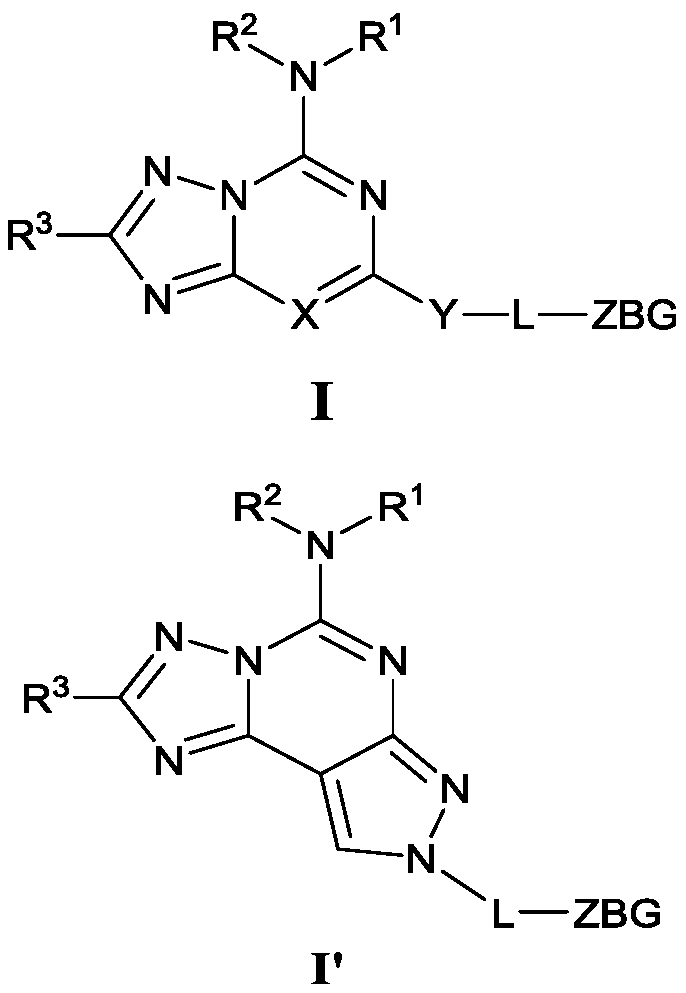
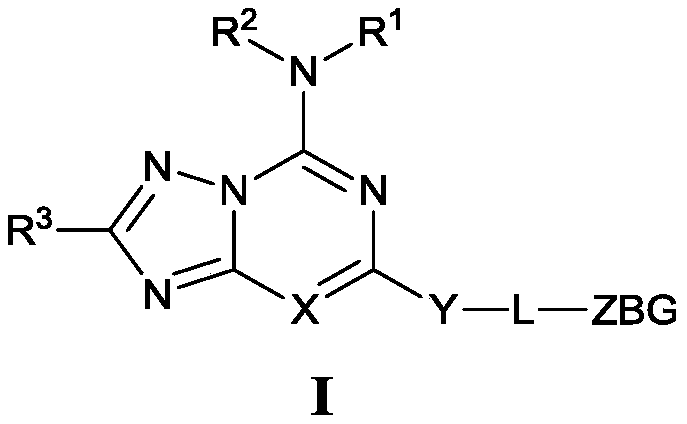
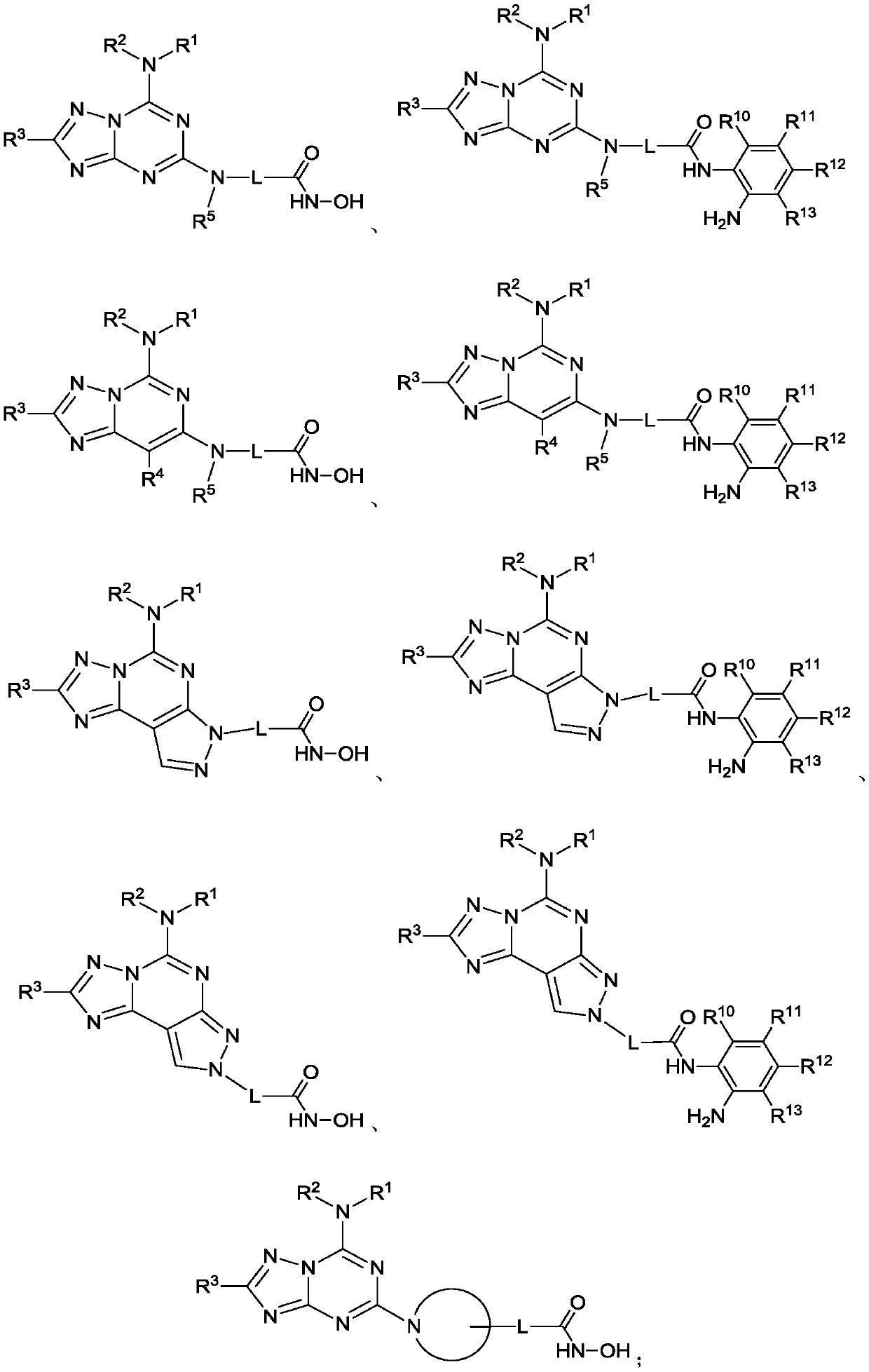
Triazolocyclic compound, and preparation method, intermediate and application thereof
A compound and solvate technology, applied in the field of triazolo ring compounds, can solve problems such as unreported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0275] Example 1: 4-(((7-amino-2-(furan-2-yl-[1,2,4]triazol[1,5-a][1,3,5]triazine-5- Base) amino) methyl)-N-hydroxybenzamide (compound I-1) preparation
[0276] Step 1: 4-(((7-amino-2-(furan-2-yl-[1,2,4]triazol[1,5-a][1,3,5]triazin-5-yl )amino)methyl)-Preparation of methyl N-hydroxybenzoate (intermediate Int-1)
[0277]
[0278] 2-(furan-2-yl)-5-methylsulfonyl-[1,2,4]triazol[1,5-a][1,3,5]triazin-7-amine (0.10g, 0.36mmol) (see J Med Chem, 2015, 58, 718-738 for the preparation method) and methyl 4-aminomethylbenzoate (0.248g, 1.5mmol) were dissolved in acetonitrile (10mL), and stirred overnight at room temperature. The solvent was evaporated under reduced pressure, and the remaining solid was separated and purified by silica gel column chromatography to obtain a white solid intermediate Int-1 (0.124 g, yield 95%). 1 H NMR (500MHz, DMSO-d 6 )δ8.25(s,2H),8.11–8.02(m,1H),7.92(d,J=8.1Hz,2H),7.86(s,1H),7.46(d,J=7.9Hz,2H), 7.10–6.99 (m, 1H), 6.67 (s, 1H), 4.64–4.52 (m, 2H), 3....
Embodiment 2
[0282] Example 2: 4-(2-((7-amino-2-(furan-2-yl-[1,2,4]triazol[1,5-a][1,3,5]triazine- Preparation of 5-yl)amino)ethyl)-N-hydroxybenzamide (compound 1-2)
[0283]
[0284] Replace the "methyl 4-aminomethylbenzoate" in Step 1 of Example 1 with "methyl 4-(2-aminoethyl)benzoate" (see WO2017133521 for the preparation method), and the rest of the required raw materials, reagents and The preparation method is the same as in Example 1, and white solid compound (I-2) can be obtained. 1 H NMR (500MHz, DMSO-d 6 )δ11.15(s,1H),8.99(s,1H),8.21(s,2H),7.87(s,1H),7.69(d,J=7.8Hz,2H),7.62–7.49(m,1H ),7.37–7.29(m,2H),7.06(d,J=3.2Hz,1H),6.68(s,1H),3.55–3.44(m,2H),2.95–2.83(m,2H); HRMS( ESI) C 17 h 17 N 8 o 3 + [M+H] + Calculated: 381.1424; Found: 381.1428.
Embodiment 3
[0285] Example 3: 4-(3-((7-amino-2-(furan-2-yl-[1,2,4]triazol[1,5-a][1,3,5]triazine- Preparation of 5-yl)amino)propyl)-N-hydroxybenzamide (compound I-3)
[0286]
[0287] Replace "4-aminomethylbenzoic acid methyl ester" in Example 1 with "4-(3-aminopropyl) benzoic acid methyl ester" (see WO2012117421 for the preparation method), and the remaining required raw materials, reagents and preparation methods Same as Example 1, white solid compound (I-3) can be obtained. 1 H NMR (800MHz, DMSO-d 6 )δ11.13(s,1H),8.96(s,1H),8.50–7.91(m,2H),7.87(s,1H),7.68(d,J=8.0Hz,2H),7.61–7.49(m ,1H),7.31(d,J=7.9Hz,2H),7.07–7.03(m,1H),6.68(s,1H),3.32–3.25(m,2H),2.68(t,J=7.7Hz, 2H),1.89–1.81(m,2H); HRMS(ESI)C 18 h 19 N 8 o 3 + [M+H] + Calculated: 395.1580, Found: 395.1561.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com