Difluoromethyl selenate compound and synthesis method thereof
A synthesis method and compound technology, which are applied in the production of steroids, organic chemistry, bulk chemicals, etc., can solve problems such as unreported synthesis methods of difluoromethylselenoate compounds, and achieve environmental friendliness, mild conditions, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
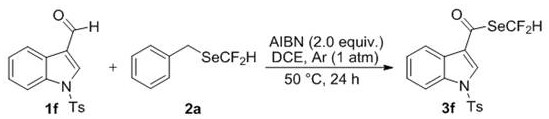
Examples
Embodiment 1
[0027] Operation steps: add 0.75 mmol p-tolualdehyde 1a, 0.5 mmol benzylselenide difluoromethyl compound 2a and 1.0 mmol AIBN in a 10 mL single-necked flask, seal the rubber stopper and evacuate, and replace the air in the bottle with an argon balloon , so that the bottle is filled with argon. Then, 1,2-dichloroethane (2.5 mL) was added to the flask through a syringe under an argon atmosphere, and reacted at 50°C for 24 h. After the reaction, the solvent was removed by rotary evaporation under reduced pressure, and the residue was purified by flash silica gel column to obtain the corresponding difluoromethylselenide compound 3a with a yield of 90%.
[0028]
[0029] Se Yellow solid; Melting point: 46-47 o C; Eluant: ethyl acetate / petroleum ether (1:100,R f = 0.30). 1 H NMR (400 MHz, CDCl 3 ) δ 7.71 (d, J = 8.2 Hz, 2H), 7.61 (t, J =53.6 Hz, 1H), 7.30 (d, J = 8.1 Hz, 2H), 2.43 (s, 3H); 19 F NMR (376 MHz, CDCl 3 ) δ -96.08 (d, J = 53.6 Hz, 2F); 13 C NMR (101 M...
Embodiment 2
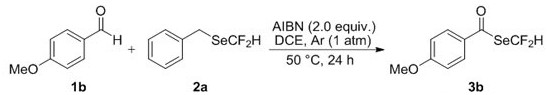
[0031] Operation steps: Add 0.75 mmol 4-methoxybenzaldehyde 1b, 0.5 mmol benzyldifluoromethylselenium compound 2a and 1.0 mmol AIBN to a 10 mL single-necked flask, seal it with a rubber stopper, and replace the bottle with an argon balloon The air, so that the bottle is filled with argon. Then, 1,2-dichloroethane (2.5 mL) was added to the flask through a syringe under an argon atmosphere, and reacted at 50°C for 24 h. After the reaction, the solvent was removed by rotary evaporation under reduced pressure, and the residue was purified by flash silica gel column to obtain the corresponding difluoromethylselenide compound 3b with a yield of 75%.
[0032]
[0033] Se Yellow solid; Melting point: 38-39 o C. Eluant: ethyl acetate / petroleum ether (1:30,R f = 0.30). 1 H NMR (400 MHz, CDCl 3 ) δ 7.77 (d, J = 8.9 Hz, 2H), 7.60 (t, J =53.6 Hz, 1H), 6.95 (d, J = 8.9 Hz, 2H), 3.88 (s, 3H); 19 F NMR (376 MHz, CDCl 3 ) δ -95.95 (d, J = 53.6 Hz, 2F); 13 C NMR (101 MHz, CD...
Embodiment 3
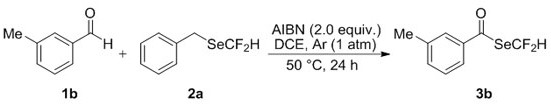
[0035] Operation steps: add 0.75 mmol 3-methylbenzaldehyde 1b, 0.5 mmol benzyldifluoromethylselenide 2a and 1.0 mmol ADVN to a 10 mL single-necked flask, seal the rubber stopper and evacuate it, and replace the Air, so that the bottle is filled with argon. Then, 1,2-dichloroethane (2.5 mL) was added to the flask through a syringe under an argon atmosphere, and reacted at 50°C for 24 h. After the reaction, the solvent was removed by rotary evaporation under reduced pressure, and the residue was purified by a flash silica gel column to obtain the corresponding difluoromethylselenide compound 3b with a yield of 63%.
[0036]
[0037] Se Yellow liquid; Eluant: ethyl acetate / petroleum ether (1:100, R f = 0.30). 1 HNMR (400 MHz, CDCl 3 ) δ 7.61 (t, J = 53.6 Hz, 1H), 7.61 – 7.60 (m, 2H), 7.48 –7.45 (m, 1H), 7.38 (t, J = 7.9 Hz, 1H), 2.43 (s, 3H); 19 F NMR (376 MHz, CDCl 3 ) δ -96.14 (d, J = 53.6 Hz, 2F); 13 C NMR (101 MHz, CDCl 3 ) δ 191.2 (t, J =2.7 Hz), 139.5, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com