A class of blue organic semiconductor materials based on 2,6-di-tert-butylanthracene and its preparation method and application
An organic semiconductor, di-tert-butylanthracene technology, applied in the field of blue organic semiconductor materials and its preparation, can solve the problems of low fluorescence quantum yield, weakened fluorescence, poor stability, etc., and achieve excellent comprehensive performance and stable material structure , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
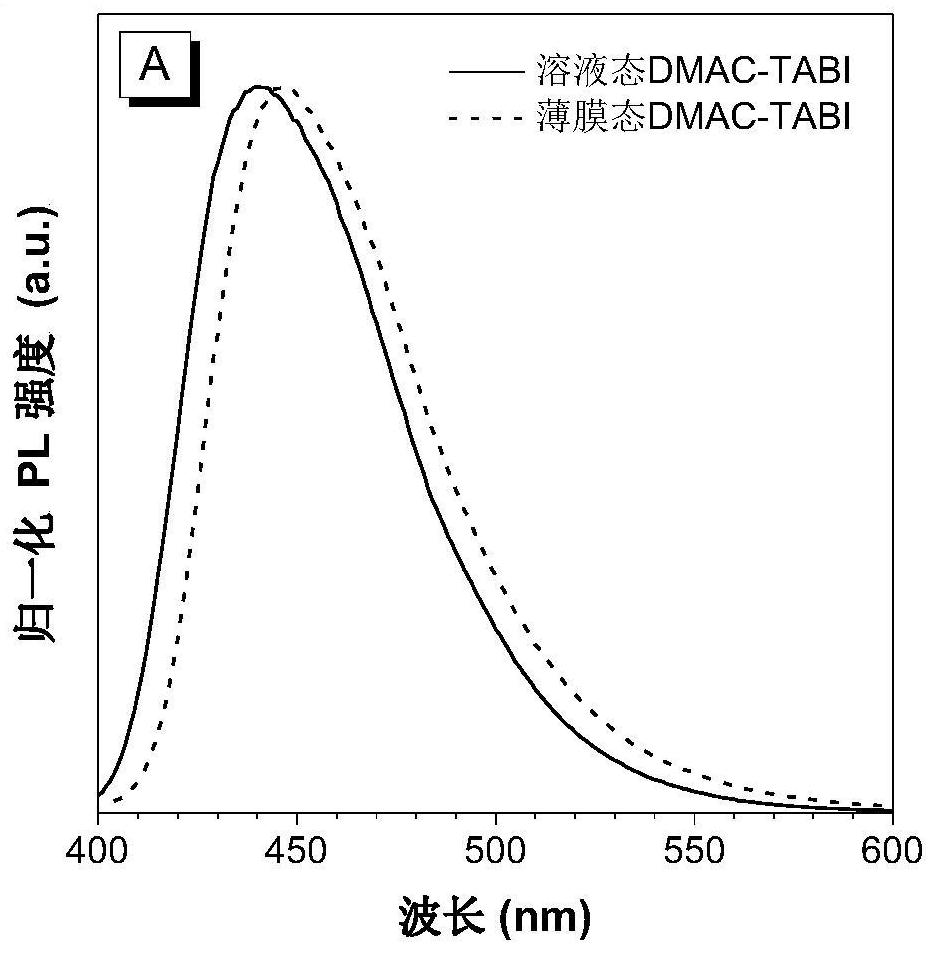
[0042] Preparation of Blue Light-emitting Organic Semiconductor Materials Based on 2,6-Di-tert-butylanthracene (DMAC-TABI)
[0043]
[0044] Reaction equation (1):
[0045]
[0046]
[0047] (1) Synthesis of Intermediate 2: In a reaction flask containing anthracene (4.45g, 25mmol), add 25mL of trifluoroacetic acid (TFA), and add tert-butanol (8.5ml, 88.8mmol) dropwise to the reaction In the bottle, after the dropwise addition, heat and reflux for 12 hours. After the reaction, pour the reaction solution into cold water to quench, stir and precipitate, then carry out suction filtration, then stir and reflux with ethanol and a small amount of n-hexane for recrystallization, and obtain the preliminary product by suction filtration Then recrystallize with ethanol twice to obtain a white solid product (intermediate 2), with a yield of 76%;
[0048] (2) Synthesis of Intermediate 3: Add Intermediate 2 (1.45g, 5mmol) into the reaction flask, dissolve it with dichloromethane (...
Embodiment 2
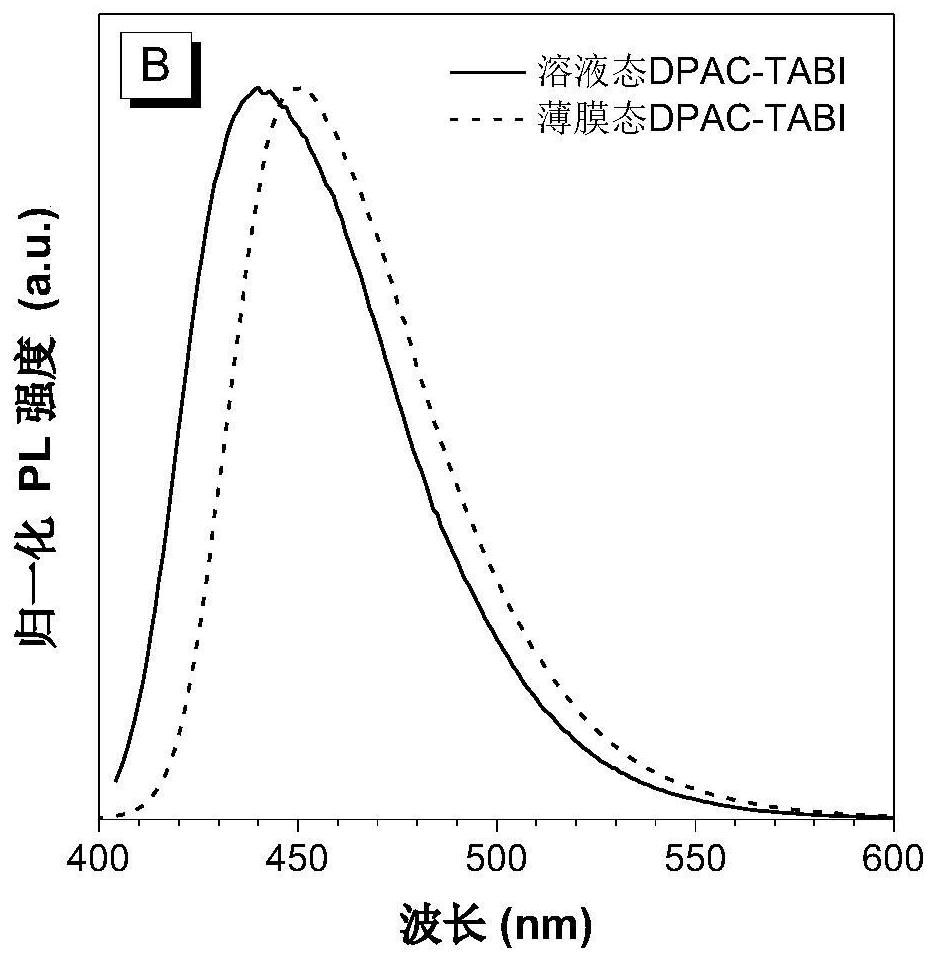
[0052] Preparation of Blue Light-emitting Organic Semiconductor Material (DPAC-TABI) Based on 2,6-Di-tert-butylanthracene
[0053]
[0054] Reaction equation (2):
[0055]
[0056] (1) with embodiment 1;
[0057] (2) with embodiment 1;
[0058] (3) with embodiment 1;
[0059] (4) Synthesis of DPAC-TABI: intermediate 5 (0.78g, 1mmol), intermediate 6 (0.60g, 1.5mmol), tetrakis(triphenylphosphine) palladium (0.12g, 0.1mmol), potassium carbonate ( Add 0.42g, 4mmol) into the reaction flask, replace the nitrogen three times, inject the solvent (toluene:ethanol:water=8:1:1 (volume ratio)) under the protection of nitrogen, heat and reflux at 110°C for 12h after the injection is completed, and the reaction is complete After extraction, concentration, powdering and column purification, a white solid product (DPAC-TABI) was obtained with a reaction yield of 78%. 1 H NMR (500MHz, CDCl 3 ), δ(TMS,ppm):8.03–7.90(m,2H),7.88–7.74(m,4H),7.65–7.44(m,15H),7.34(t,J=6.0Hz,4H),7.30( dd,...
Embodiment 3
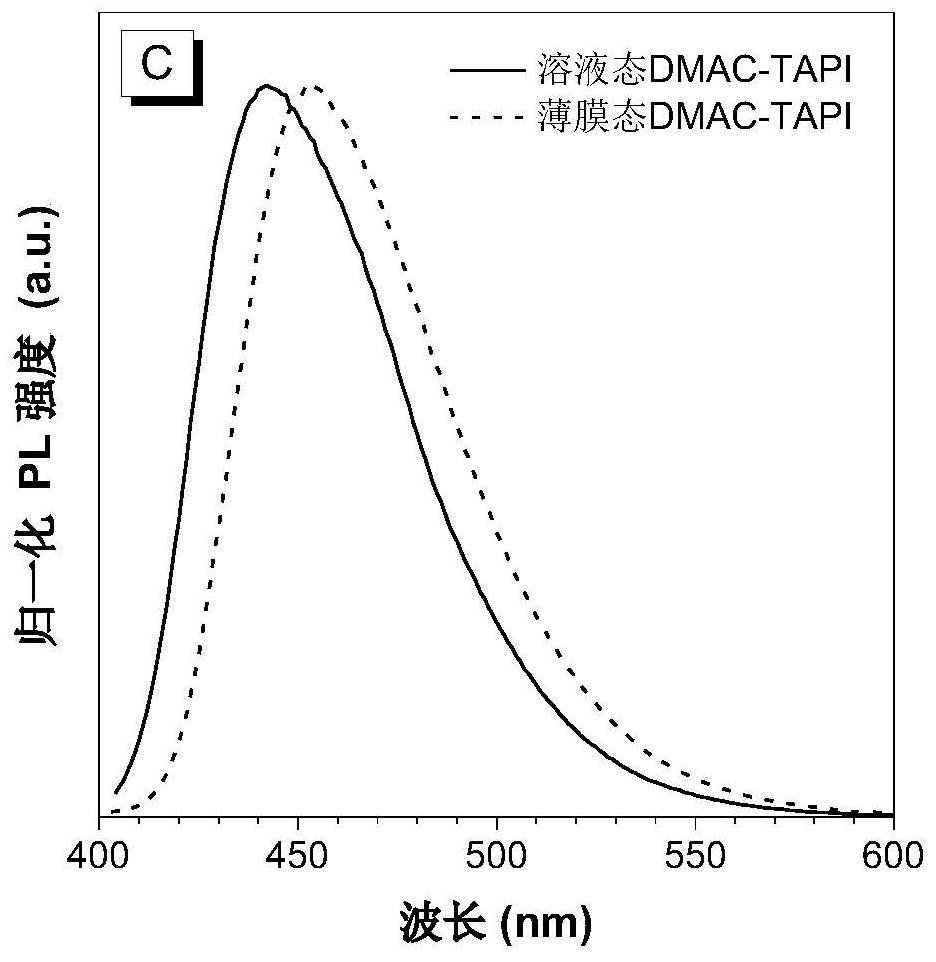
[0061] Preparation of Blue Light-emitting Organic Semiconductor Materials Based on 2,6-Di-tert-butylanthracene (DMAC-TAPI)
[0062]
[0063] Reaction equation (3):
[0064]
[0065] (1) with embodiment 1;
[0066] (2) with embodiment 1;
[0067] (3) with embodiment 1;
[0068] (4) Synthesis of DMAC-TAPI: intermediate 5 (0.65g, 1mmol), intermediate 6 (0.75g, 1.5mmol), tetrakis(triphenylphosphine) palladium (0.12g, 0.1mmol), potassium carbonate ( Add 0.42g, 4mmol) into the reaction flask, replace the nitrogen three times, inject the solvent (toluene:ethanol:water=8:1:1 (volume ratio)) under the protection of nitrogen, heat and reflux at 110°C for 12h after the injection is completed, and the reaction is complete After extraction, concentration, powdering and column purification, a white solid product (DMAC-TAPI) was obtained with a reaction yield of 73%. 1 H NMR (500MHz, CDCl 3 ), δ(TMS,ppm):8.97(d,J=7.4Hz,1H),8.79(dd,J=31.6,8.5Hz,2H),7.87(d,J=9.8Hz,1H),7.85–7.77 (m,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com