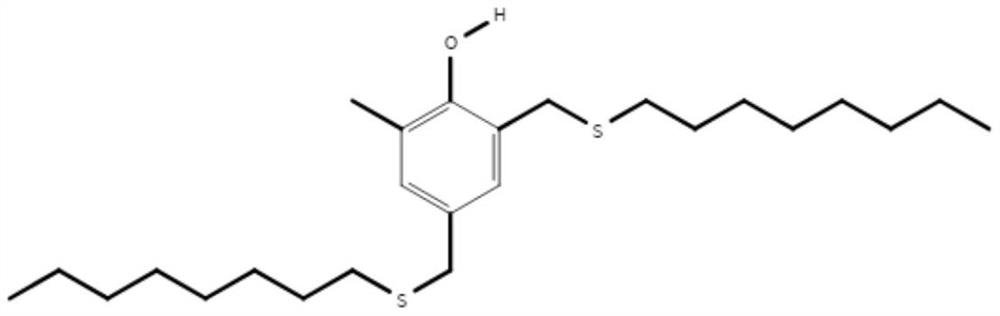
A kind of preparation method of 2,4-two (n-octyl sulfide methylene)-6-methylphenol
A technology of n-octylsulfamethylene and cresol, which is applied in the field of preparation of phenolic antioxidants, can solve problems such as long reaction time, unfavorable reactions, and reduced yields, and achieve simplified production operations and improved reaction Efficiency, the effect of reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Replace the reaction flask with stirrer, condenser and thermometer with nitrogen, add 34.7g o-cresol, 89.5g n-octyl mercaptan and 36.5g paraformaldehyde therein, and add 40% dimethylamine aqueous solution 340g and 8.5 g sodium didodecyl diphenyl ether disulfonate, under stirring conditions, raise the temperature to 100°C to reflux, the reaction solution forms a uniform emulsion state, and continue to react for 5 hours. After the reaction is finished, the temperature of the reaction system is lowered by 5° C., and left to stand for 10 hours, the crystallization of the antioxidant 1520 2,4-bis(n-octylthiomethylene)-6-methylphenol is cooled in the reaction system, and the obtained White powdery product, kept at a low temperature of 5-10°C, filtered to separate the solid powder, washed with ethanol solution, and dried to obtain the target compound 2,4-bis(n-octylthiomethylene)-6-methylphenol 127.7 g, its reaction yield is 97.1%, and the product purity is 98.8%.
Embodiment 2
[0020] Replace the reaction flask with stirrer, condenser and thermometer with nitrogen, add 34.7g o-cresol, 135g n-octyl mercaptan and 36.5g paraformaldehyde therein, and add 40% dimethylamine aqueous solution 340g and 8.5g Didodecyl diphenyl ether sodium disulfonate, under the condition of stirring, raise the temperature to 100°C to reflux, the reaction liquid forms a uniform emulsion state, and continue to react for 6 hours. After the reaction is finished, the temperature of the reaction system is lowered by 5° C., and left to stand for 12 hours, the crystallization of the antioxidant 1520 2,4-bis(n-octylthiomethylene)-6-methylphenol is cooled in the reaction system, and the obtained White powdery product, kept at a low temperature of 5-10°C, filtered to separate the solid powder, washed with ethanol solution, and dried to obtain the target compound 2,4-bis(n-octylthiomethylene)-6-methylphenol 127.0 g, its reaction yield is 96.5%, and the product purity is 98.0%.
Embodiment 3
[0022] Replace the reaction flask with stirrer, condenser and thermometer with nitrogen, add 34.8g o-cresol, 89.5g n-octyl mercaptan and 46.2g paraformaldehyde therein, and add 280g and 7g of 33% dimethylamine aqueous solution Didodecyl diphenyl ether sodium disulfonate, under the condition of stirring, raise the temperature to 100°C to reflux, the reaction liquid forms a uniform emulsion state, and continue to react for 4 hours. After the reaction is finished, the temperature of the reaction system is lowered by 10° C., and left to stand for 10 hours, the crystallization of the antioxidant 1520 2,4-bis(n-octylthiomethylene)-6-methylphenol that is cooled down is generated in the reaction system to obtain White powdery product, kept at a low temperature of 5-10°C, filtered to separate the solid powder, washed with ethanol solution, and dried to obtain the target compound 2,4-bis(n-octylthiomethylene)-6-methylphenol 126.2 g, its reaction yield is 96.0%, and the product purity is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com