Preparation method of brexpiprazole tablets
A technology of breiprazole tablets and breiprazole, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve problems such as difficulty in improving the dissolution rate, and achieve high temperature suppression Degradation, ensuring drug stability, and reducing the effect of extrusion temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The prescription technology of embodiment 1-5 is shown in Table 1, and the unit of all components is: g.
[0028] Table 1
[0029]
[0030] Preparation:
[0031] Potassium citrate, sorbitol and brepiprazole are heated and melted in a hot-melt extruder, and then the melted material is extruded and granulated, and directly compressed with mannitol, calcium hydrogen phosphate, micropowder silica gel and magnesium stearate .
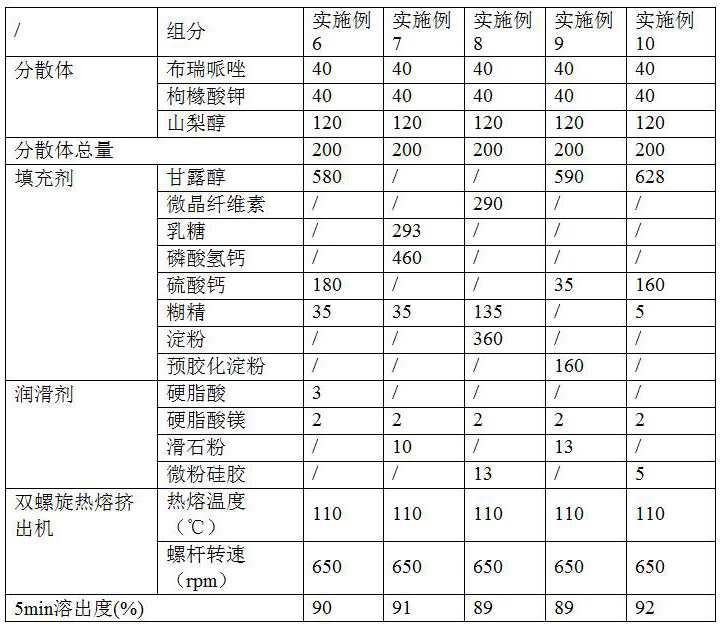
[0032] Examples 6-10 investigated the types and dosages of fillers and lubricants for each component. The formulation process is shown in Table 2, and the unit of the components in the table is g.
[0033] Table 2
[0034]
[0035] Preparation:
[0036] Potassium citrate, sorbitol and brepiprazole were heated and melted in a hot-melt extruder, and then the melt was extruded into pellets, mixed with the corresponding excipients in each example, and then directly compressed into tablets.
[0037] It can be seen that different fillers are sele...
Embodiment 11
[0039] In the published patent CN107397730A embodiment 2-1, 2-2, 2-3 and embodiment 1, embodiment 2, embodiment 3 in the present invention, in vitro dissolution curve investigation, such as figure 1 ,table 3.
[0040] In vitro dissolution test conditions: Dissolution test method (paddle method, 50rpm), using disodium hydrogen phosphate-citric acid buffer solution (900ml) with a pH value of 4.5 as the test liquid.
[0041] table 3
[0042]
[0043] It can be seen that the present invention heats and melts potassium citrate, sorbitol and brepiprazole in a hot-melt extruder, then extrudes the molten material to granulate, mixes it with the corresponding auxiliary materials in each embodiment, and directly compresses it into tablets , the 5min dissolution rate of the prepared tablet is higher than the dissolution rate of the currently published patents and dosage forms reported in the literature. Therefore, the bripiprazole is made into a solid dispersion, which greatly improv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com