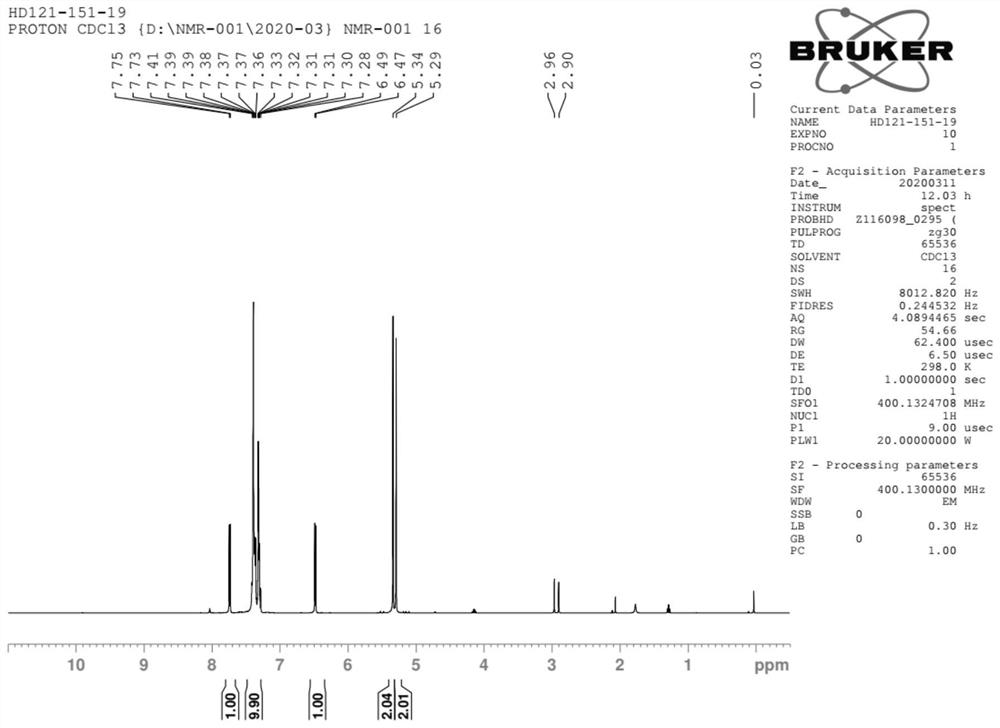
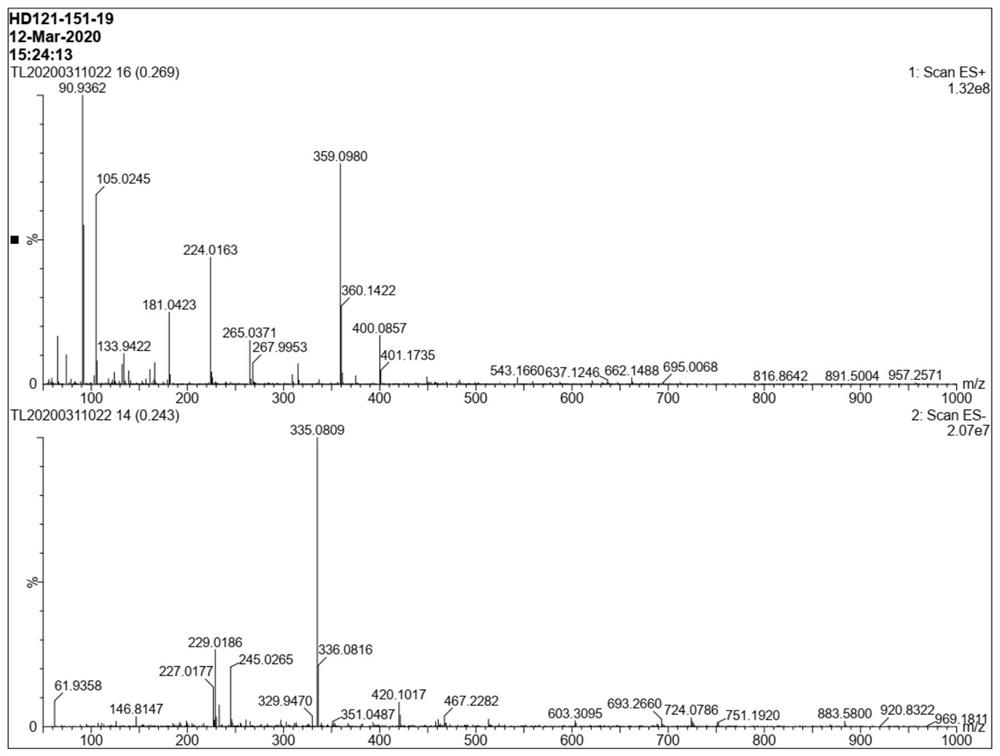
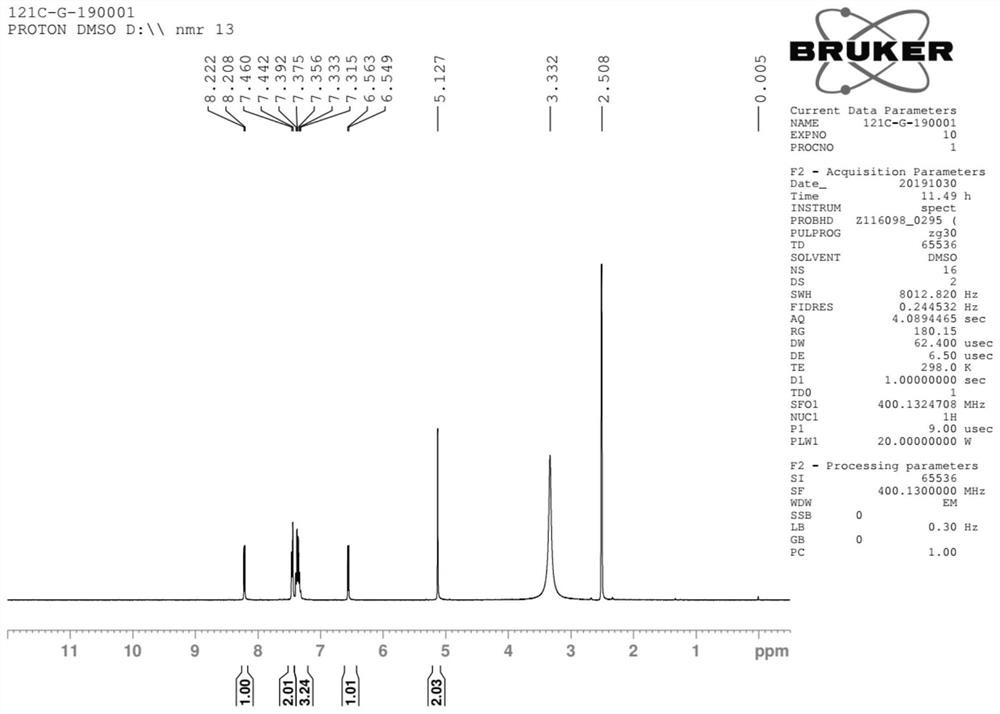
Method for preparing baloxavir intermediate
A technology for intermediates and compounds is applied in the field of preparation of baloxavir intermediates of anti-influenza drugs, can solve problems such as being unsuitable for industrial production, serious environmental pollution, environmental pollution, etc., and achieves low cost, high safety factor, and avoidance of Effects of use under high temperature conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0050] Synthesis of compounds of formula II
[0051]
[0052] Acetone (200g), H 2 O (250g) was added to a 10L reaction flask, followed by adding the compound of formula I (50g, 1.0eq), TEMPO (11.0g, 0.2eq), sodium bicarbonate (44g, 1.5eq), KBr (8.2g, 0.2eq ). Slowly cool down to 0°C. Add sodium hypochlorite (520g, 2.0eq) solution dropwise under temperature control at 0-10°C, after the dropwise addition, keep warm at 5-10°C for 30min, monitor by liquid phase HPLC, and the reaction is complete. Keep warm at 5-10°C, add Na 2 SO 3 (8g, 0.2eq) quenched and stirred for 0.5h. Acetone was distilled off under reduced pressure at 40-50°C. The impurities were extracted twice with dichloromethane (200g*2), and the aqueous phase was transferred to a kettle. The kettle was kept warm at 15-20°C, and 12N HCl was added dropwise until pH = 1-2. After beating at 25°C for 10 hours, filtered, the filter cake was rinsed twice with 25°C water, and the crude product was dried at 60°C for 10...
Embodiment 3
[0060] Synthesis of compounds of formula II
[0061]
[0062] Dichloromethane (20g), H 2 O (25g) was added to a 10L reaction flask, followed by adding the compound of formula I (5g, 1.0eq), TEMPO (0.28g, 0.05eq), sodium bicarbonate (4g, 1.5eq), KBr (0.8g, 0.2eq ). Slowly cool down to 0°C. Add sodium hypochlorite (52g, 2.0eq) solution dropwise under temperature control at 0-10°C, after the dropwise addition, keep warm at 5-10°C for 30min, monitor by liquid phase HPLC, and the reaction is complete. Keep warm at 5-10°C, add Na 2 SO 3 (0.8g, 0.2eq) quenched and stirred for 0.5h. Separate the layers and transfer the aqueous phase to a kettle. Keep the kettle warm at 15-20°C, add 12N HCl dropwise until pH = 1-2. After beating at 25°C for 10 hours, filter, rinse the filter cake twice with 25°C water, and dry the crude product at 60°C for 10 hours to obtain compound 4.2 of formula II g, yield 76%
[0063] Synthesis of compounds of formula III
[0064]
[0065] Methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com