Synthesis method of poly 6-hydroxyhexanoate
A technology of hydroxycaproic acid ester and synthesis method, which is applied in the chemical industry, can solve the problems of unstable production process and unsatisfactory yield of 6-hydroxycaproic acid ester, and achieve the effects of fast reaction progress, good production effect and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
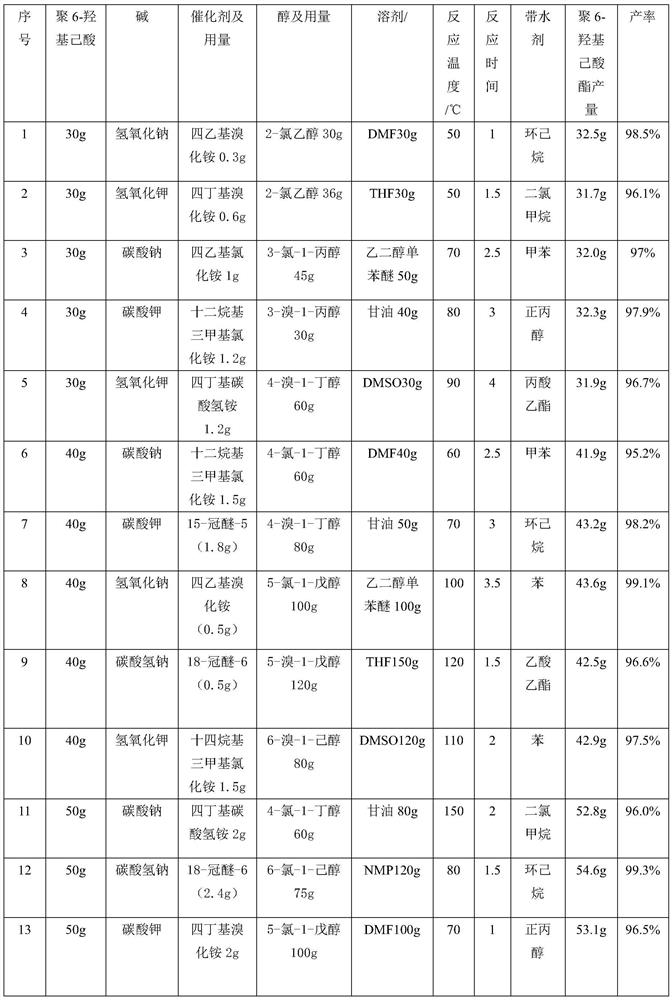
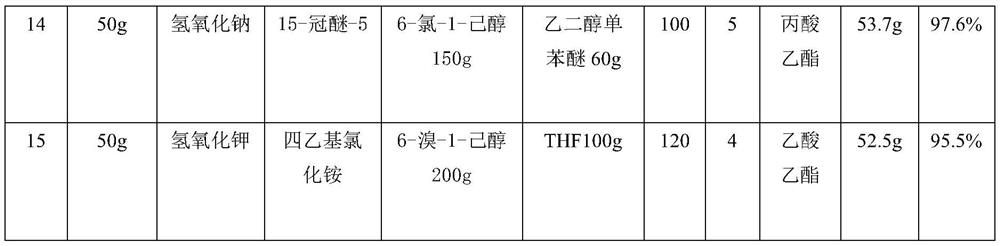
[0019] Neutralize 30g of poly-6-hydroxycaproic acid with 0.1mol / L sodium hydroxide solution to neutrality, then add 30g of 2-chloroethanol, add DMF30g, mix and place in a 250ml round bottom flask, add at 50°C 0.3 g of tetraethylammonium bromide is placed in a collector-type constant temperature heating magnetic stirrer to obtain poly-6-hydroxycaproic acid ester through 1 h of esterification, and then add 20 ml of cyclohexane to the obtained poly-6-hydroxycaproic acid ester Alkanes were removed to obtain 32.5 g of poly-6-hydroxycaproic acid ester, and the yield of poly-6-hydroxycaproic acid ester was 98.5%.
Embodiment 2-5
[0021] The quality of poly-6-hydroxycaproic acid is the same as in Example 1, and the others are different from Example 1, see Table 1 for details.
Embodiment 6-10
[0023] The mass of poly-6-hydroxycaproic acid is 40g, and the others are different from Example 1, see Table 1 for details.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com