Preparation method of poly-6-hydroxyhexanoate
A technology of hydroxycaproic acid ester and hydroxycaproic acid, applied in the chemical industry, can solve the problems of unstable production process and unsatisfactory 6-hydroxycaproic acid yield, and achieve good production effect, high product quality and process safety factor. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
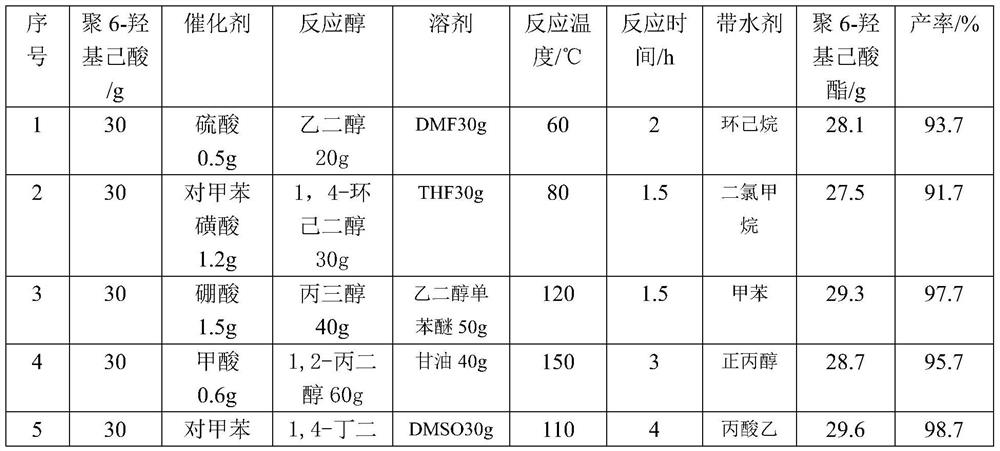
[0016] Mix 30g of poly-6-hydroxycaproic acid and 20g of ethylene glycol in a 250ml round-bottomed flask, add 0.5g of sulfuric acid at 60°C and place it in a collector type constant temperature heating magnetic stirrer for 2 hours to obtain poly Poly 6-hydroxycaproic acid ester, then add 20ml cyclohexane in the obtained poly 6-hydroxycaproic acid ester, remove water to obtain poly 6-hydroxycaproic acid ester 28.1g, poly 6-hydroxycaproic acid ester yield 93.7 %.
Embodiment 2-5
[0018] The quality of poly-6-hydroxycaproic acid is the same as in Example 1, and the others are different from Example 1, see Table 1 for details.
Embodiment 6-10
[0020] The mass of poly-6-hydroxycaproic acid is 40g, and the others are different from Example 1, see Table 1 for details.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com