Detection method of nitrosamine impurities in candesartan cilexetil
A technology of candesartan cilexetil and detection method, applied in the field of drug analysis, to achieve the effect of ensuring drug safety and good system adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In order to describe the technical solution of the present invention more clearly and completely, the present invention will be further described in detail through specific examples below. It should be understood that the specific examples described here are only used to explain the present invention, and are not used to limit the present invention. Various changes can be made within the scope defined by the rights of the present invention.
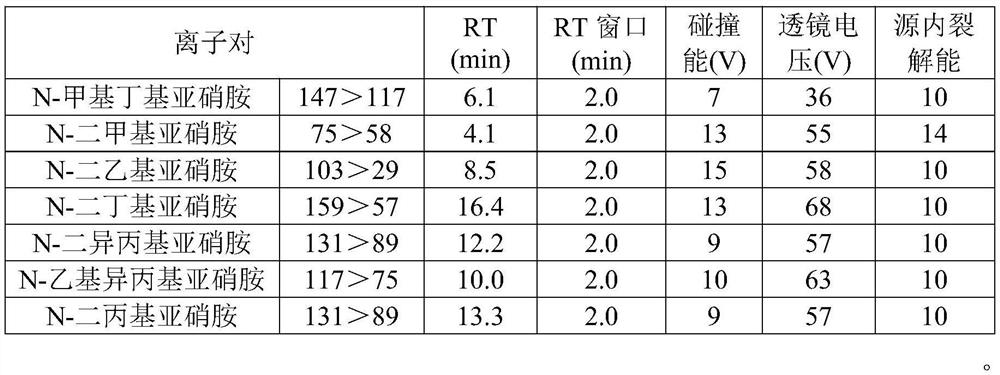
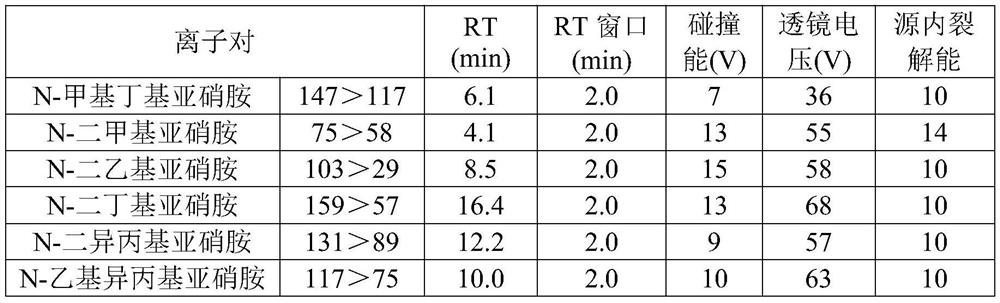
[0029] The reagent information that the present invention adopts is as follows:
[0030] The sample candesartan cilexetil was collected from Tiandi Hengyi Pharmaceutical Co., Ltd., the batch number is 190901;
[0031] The content of the reference substance N-methylbutyl nitrosamine (NMBA) is 98%; the content of N-dimethyl nitrosamine (NDMA) is 99.2%; the content of N-diethyl nitrosamine (NDEA) It is 99.6%; The content of N-dibutyl nitrosamine (NDBA) is 99%; The content of N-diisopropyl nitrosamine (NDIPA) is 99.8%; N-ethyl isoprop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com