Synthetic method of abiraterone acetate and intermediate thereof
A technology of abiraterone acetate and a synthesis method, which is applied in chemical instruments and methods, steroids, androstane derivatives, etc., can solve the problems of difficult control of synthesis process, complicated reaction, low yield of key steps, etc. The effect of reducing the probability of side reactions, improving yield and purity, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] One embodiment of the present invention provides a method for synthesizing an abiraterone acetate intermediate, comprising the following step S10.
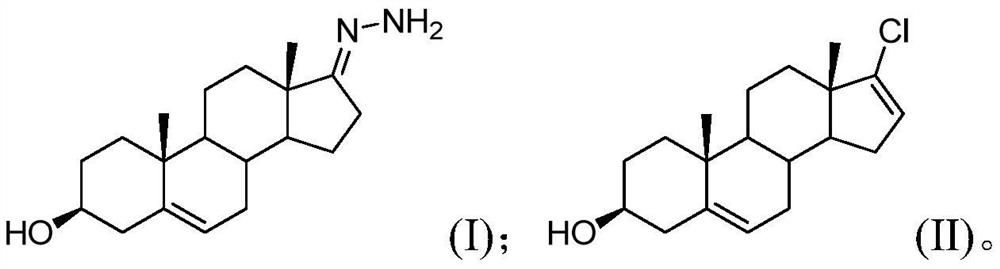
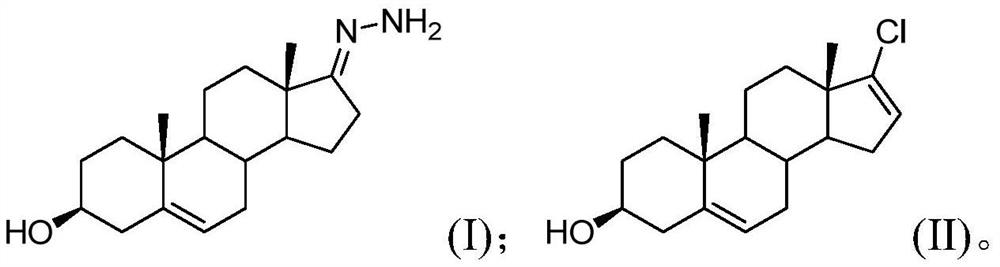
[0036] Step S10, performing a chlorination reaction on the compound of formula (I), a chlorinating agent and a base to obtain an intermediate of abiraterone acetate; wherein, the mass ratio of the compound of formula (I) to the base is 1:(1.5~3); (I) The structures of the compound and the Abiraterone acetate intermediate are as follows:
[0037]
[0038] Formula (I) compound, chlorination reagent and alkali are carried out chlorination reaction, obtain the abiraterone acetate intermediate; Wherein the mass ratio of control formula (I) compound and alkali is 1:(1.5~3); The chlorination reagent The reaction activity is high. At the same time, by controlling the mass ratio of the compound of formula (I) and the base to 1: (1.5-3), the selectivity of the chlorination reaction is improved, so that the chlorination reaction ca...
Embodiment 1
[0094] 1) Put 400g of absolute ethanol, 72g of 80% hydrazine hydrate, and 100g of dehydroepiandrosterone into a three-necked flask in sequence, start stirring, then raise the temperature to 35°C, add 0.5g of hydrazine sulfate, and keep the reaction at 35°C for 20 hours. Then use TLC spot plate detection, the results show that the raw materials have reacted completely, then pump the reaction solution into ice water for water analysis, centrifuge after stirring for 40 minutes, rinse the filter cake with water, dry the wet material at 50°C for 30 hours, and obtain compound Ⅰ . The calculation results showed that the yield of compound Ⅰ was 98.5%.
[0095] Yield of compound I = molar amount of compound I / molar amount of dehydroepiandrosterone × 100%
[0096] 2) Take 100g of the intermediate I obtained in step 1), dissolve it with 800g of dichloromethane, and make a reserve solution for subsequent use. Put 1000g of dichloromethane into the three-necked bottle, cool down to -5°C, ...
Embodiment 2
[0103] Example 2 is substantially the same as Example 1, except that 300 g of tetramethylguanidine is added dropwise in step 2) of Example 2, and other steps and process parameters are the same as in Example 1.
[0104] After calculation, the yield of the abiraterone acetate intermediate is 83.6%, and the purity is 99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com