4-amide substituted pyrimidine targeted DDR1 inhibitor as well as preparation and anti-tumor activity application thereof
A technology of anti-tumor drugs and small molecule inhibitors, which is applied in the field of medicinal chemistry and can solve problems such as not particularly effective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The synthesis of embodiment 1 compound
[0047] 1.1 The specific synthetic route of compound (alkali listed in route, solvent and acid-binding agent are only exemplary, not limitation of the present invention) as follows:
[0048]
[0049] 1.2 Examples of synthetic steps
[0050] Synthesis of 4-((6-amino-5-chloropyrimidin-4-yl)oxy)phenol: add substituted phenol 1.2mmol (150mg) and CsCO 3 3.0mmol (576mg) was dissolved in DMSO 25mL, and magnetite was added at the same time. Stir and heat at 80°C for half an hour to fully dissolve, then add 1.0 mmol of 4-amino-5,6-chloropyrimidine (163 mg) dissolved in 5 mL of absolute ethanol to react. Note that if it is added in a dropwise manner, in order to achieve the effect of long-term excessive reaction, the dropping time is controlled at about 1h. After reacting for 15 hours, extract with EA, remove water with anhydrous sulfuric acid, make sand, and separate by column chromatography to obtain N4-(substituted phenyl)-5,6-dich...
Embodiment 2
[0143] Embodiment 2 compound antitumor cell activity (kinase experiment)
[0144] 2.1 Experimental operation steps
[0145] (1) Prepare 1×Kinase buffer.
[0146] (2) Preparation of compound concentration gradients: the test compound concentration was 10 μM, repeated wells were detected, and a solution with a final concentration of 100 times was prepared in a 384-well plate. Then use Echo550 to transfer 250nl to the 384 reaction plate for later use. Add 250 nl of 100% DMSO to negative control wells and positive control wells, respectively.
[0147] (3) Prepare a kinase solution with a final concentration of 2.5 times with 1×Kinase buffer.
[0148] (4) Add 10 μL of 2.5-fold final concentration of kinase solution to compound wells and positive control wells; add 10 μL of 1×Kinase buffer to negative control wells.
[0149] (5) Centrifuge at 1000 rpm for 30 seconds, shake and mix well, and incubate at room temperature for 10 minutes.
[0150] (6) Use 1×Kinase buffer to prepare...
Embodiment 3
[0161] Embodiment 3 MTT method measures the inhibitory effect of target compound on tumor cell line
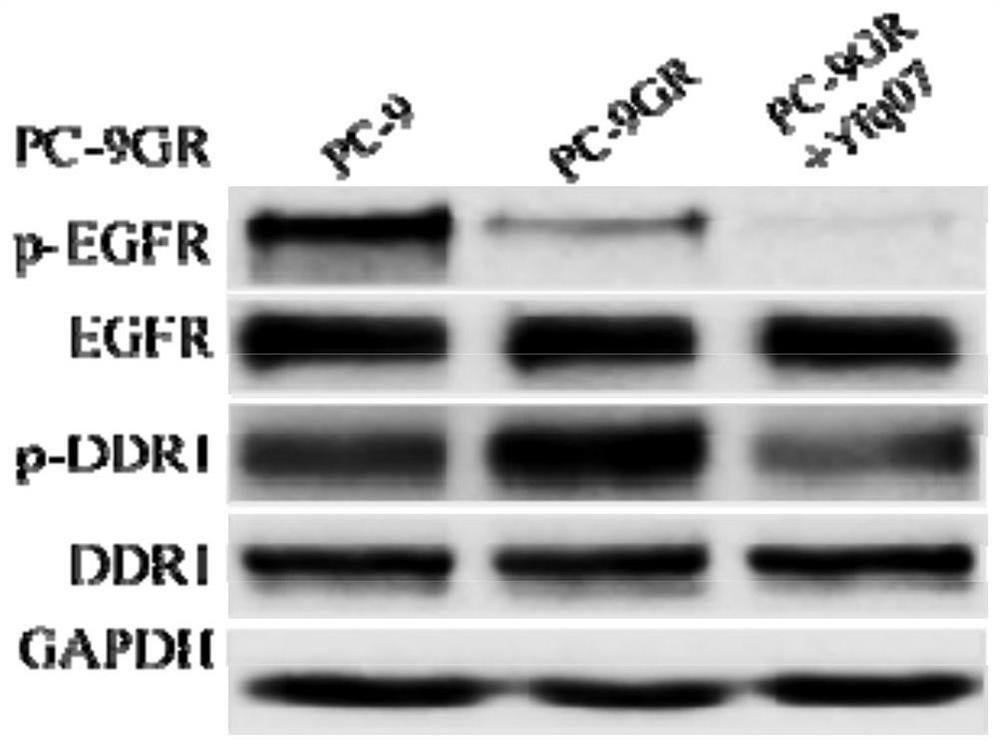
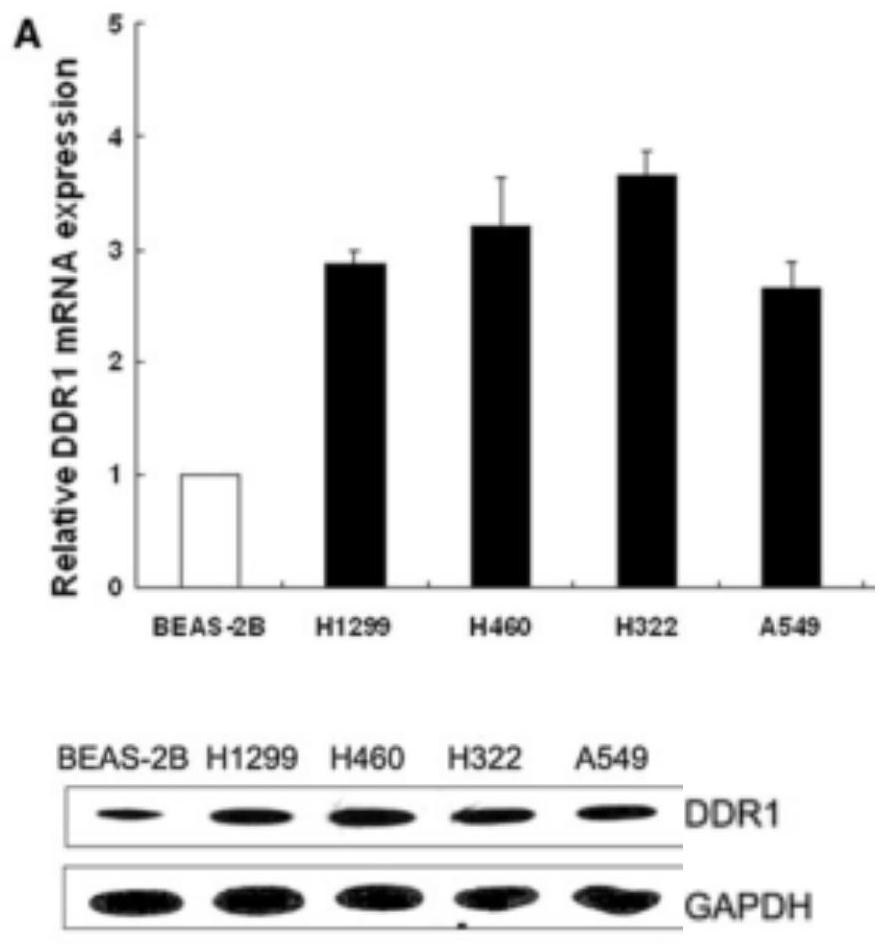
[0162] All target compounds were dissolved in DMSO and prepared as 1 mM initial solution, which was stored in a cool drug storage cabinet for future use. The initial screening concentration is 10μM, IC 50 The concentrations selected for the test were 10 μM, 5 μM, 1 μM, 0.5 μM, 0.1 μM, and 0.01 μM. First, the effect of the target compound on the proliferation of normal lung cell BEAS-2B at a concentration of 10 μM was determined by MTT method, that is, the toxic effect. Further, all compounds were screened for antitumor activity at a concentration of 10 μM, and the selected cell types included: BEAS-2B, A431, and PC-9GR. Finally, select the compound with better antiproliferative activity on these cell lines, and do IC at six concentrations of 10 μM, 5 μM, 1 μM, 0.5 μM, 0.1 μM, and 0.01 μM 50 experiment.
[0163] These cells are all adherent cells and were cultured in comple...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com