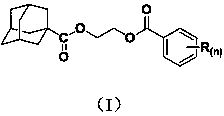
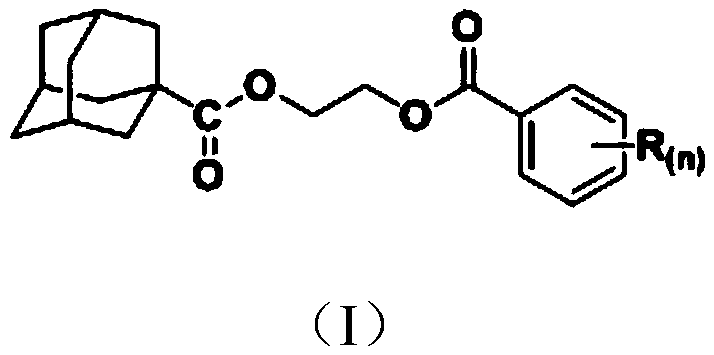
1-adamantanecarboxylic acid-2-(substituted benzoyloxy) ethyl ester compound as well as synthesis method and application thereof
A technology of adamantane carboxylate and benzoyloxy, which is applied in the field of 1-adamantane carboxylate-2-ethyl ester compounds and their synthesis, and can solve the problems of structure and biological activity that have not been reported in the literature. achieve the effect of simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthesis of embodiment 1 compound Ia (R (n)=H):
[0027] Dissolve 1.5 mmol of benzoyl chloride in 5 mL of tetrahydrofuran to prepare a tetrahydrofuran solution (5 mL) of benzoyl chloride (1.5 mmol).
[0028] The intermediate 2-hydroxyethyl-1-adamantanecarboxylate (0.336g, 1.5mmol), tetrahydrofuran (10mL) and acid-binding agent triethylamine (1.8mmol) were mixed, stirred and dissolved, and gradually dissolved in an ice bath. A solution of benzoyl chloride (1.5 mmol) in tetrahydrofuran (5 mL) was slowly added dropwise. After the dropwise addition, react at room temperature for 2 h, then filter to remove the hydrochloride of triethylamine generated by the reaction, and the filtrate is subjected to rotary evaporation to remove solvent tetrahydrofuran, and the obtained rotary evaporation residue is separated by column chromatography (eluent is a volume ratio of 1: The mixed solution of ethyl acetate and petroleum ether in 5) to obtain a light yellow oily liquid, which ...
Embodiment 2
[0031] The synthesis of embodiment 2 compound Ib (R (n)=o-fluorine):
[0032] The intermediate 2-hydroxyethyl-1-adamantanecarboxylate (0.336g, 1.5mmol), tetrahydrofuran (8.5mL) and the acid-binding agent triethylamine (2.25mmol) were mixed, stirred and dissolved, and placed in an ice bath A solution (4 mL) of o-fluorobenzoyl chloride (1.5 mmol) in tetrahydrofuran was slowly added dropwise. After the dropwise addition, react at room temperature for 3h, then filter to remove the hydrochloride of triethylamine generated by the reaction, and the filtrate is subjected to rotary evaporation to remove solvent tetrahydrofuran, and the obtained rotary evaporation residue is separated by column chromatography (eluent is a volume ratio of 1: 3 (mixture of ethyl acetate and petroleum ether) to obtain a white waxy solid, that is, 1-adamantanecarboxylic acid-2-(2-fluorobenzoyloxy)ethyl ester, and the calculated yield was 64.9%.
[0033] 1 H NMR (500MHz, CDCl 3 )δ7.97–7.90(m,1H),7.57–7.51...
Embodiment 3
[0035] Synthesis of Example 3 Compound Ic (R (n) = p-fluorine):
[0036] The intermediate 2-hydroxyethyl-1-adamantanecarboxylate (0.336g, 1.5mmol), tetrahydrofuran (8.5mL) and acid-binding agent triethylamine (2.0mmol) were mixed, stirred and dissolved, and placed in an ice bath A tetrahydrofuran solution (4 mL) of p-fluorobenzoyl chloride (1.5 mmol) was slowly added dropwise. After the dropwise addition, react at room temperature for 3h, then filter to remove the hydrochloride of triethylamine generated by the reaction, and the filtrate is subjected to rotary evaporation to remove solvent tetrahydrofuran, and the obtained rotary evaporation residue is separated by column chromatography (eluent is a volume ratio of 1: 3 mixed solution of ethyl acetate and petroleum ether) to obtain a white waxy solid, which is 2-(4-fluorobenzoyloxy)ethyl 1-adamantanecarboxylate, and the calculated yield is 55.7%.
[0037] 1 H NMR (500MHz, CDCl 3 )δ8.09–8.04(m,2H),7.16–7.10(m,2H),4.52(t,J=4....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com