Deuterated fluorene compound and light-emitting device thereof
A light-emitting device and compound technology, which is applied in the field of organic electroluminescent devices, deuterated fluorene compounds, and light-emitting devices, to achieve good film-forming properties, thermal stability, high luminous efficiency, and low driving voltage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
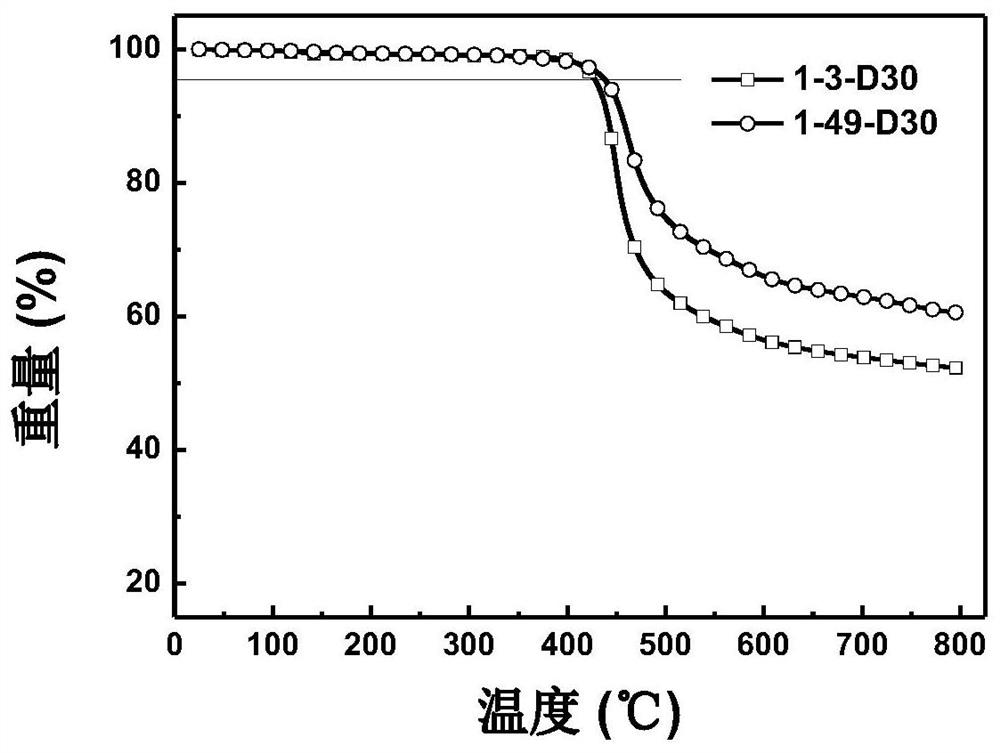
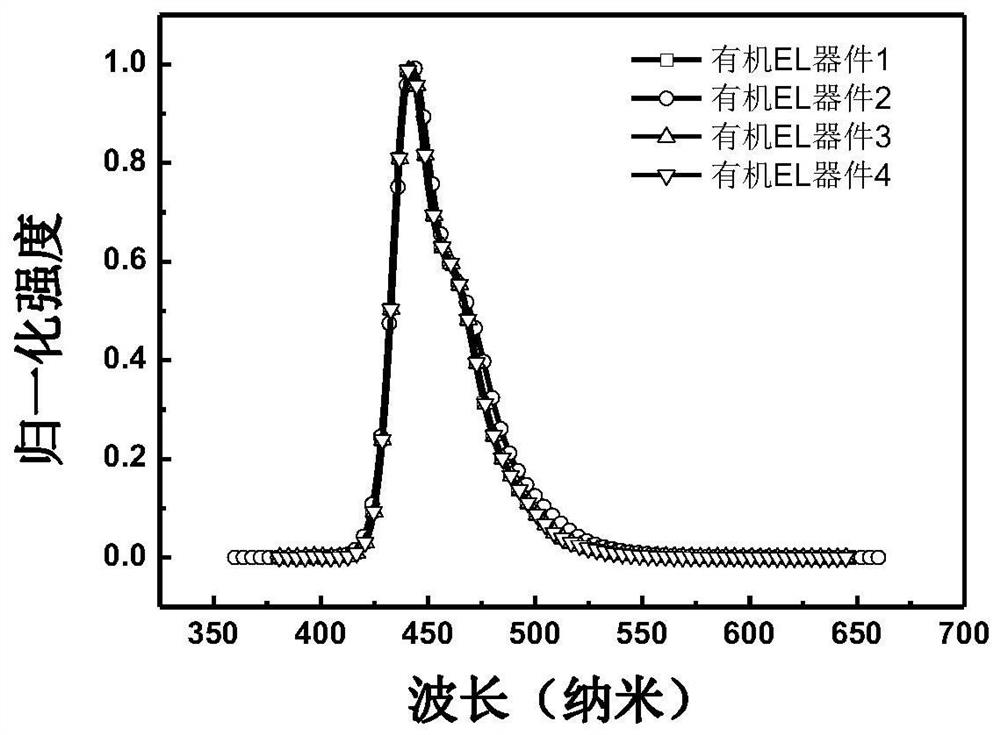
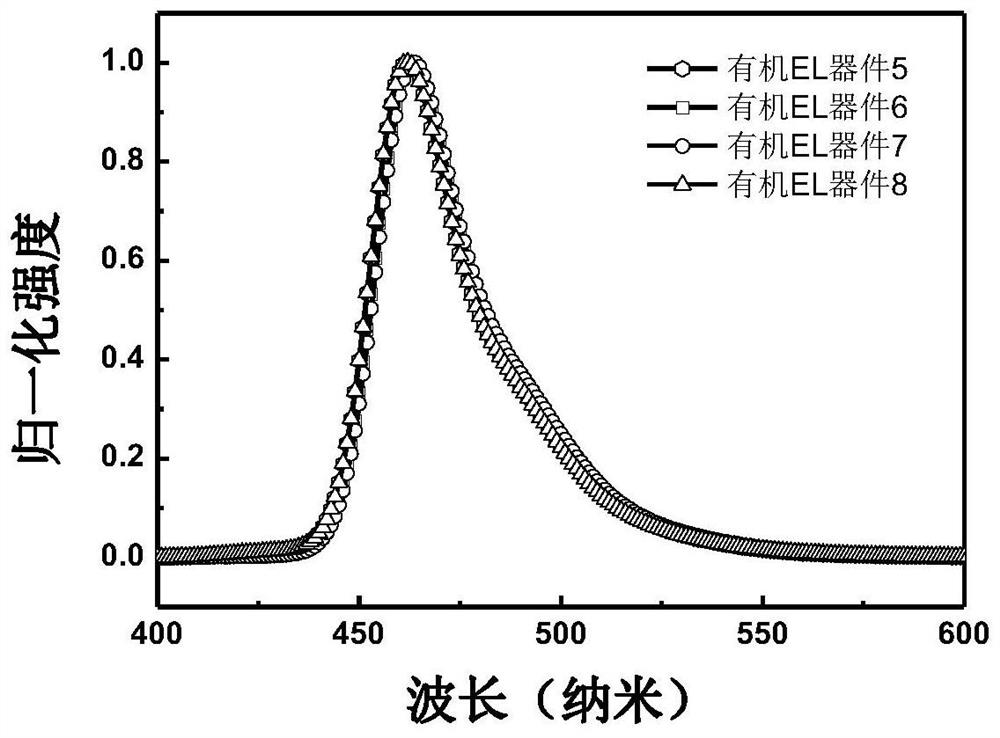
Image
Examples
Embodiment 1
[0146] Embodiment 1: the synthesis of compound 1-3-D30
[0147] [Synthesis of compound M1]
[0148] The synthetic route of compound M1 is as follows:
[0149]
[0150] Under nitrogen atmosphere, 2-(methoxycarbonyl)phenylboronic acid (8.6g, 47.8mmol), anhydrous sodium carbonate (8.4g, 79.6mmol), 1-bromo-4- Chloro-2-iodobenzene (12.6g, 39.8mmol), tetrakis(triphenylphosphine palladium) (470.8mg, 4.8mmol) and a mixed solvent of toluene, water and ethanol (100mL, toluene:water:ethanol=5:1 :1(V / V)). The system was gradually heated to reflux, and reacted overnight under reflux. After the reaction was completed, the heating was stopped and cooled to room temperature. The reaction solution was poured into water (about 200 mL), and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and further purified by column chromatography (the stationary phase was 350 mesh silica gel, and the eluent was petroleum e...
Embodiment 2
[0177] Embodiment 2: the synthesis of compound 1-2-D25
[0178] [Synthesis of compound M7]
[0179] The synthetic route of compound M7 is as follows:
[0180]
[0181] Under nitrogen atmosphere, phenylboronic acid (5.8g, 47.8mmol), anhydrous sodium carbonate (8.4g, 79.6mmol), compound M4 (12.2g, 39.8mmol), tetrakis(triphenyl Phosphine palladium) (470.8mg, 4.8mmol) and a mixed solvent of toluene, water and ethanol (100mL, toluene:water:ethanol=5:1:1 (V / V)). The system was gradually heated to reflux, and reacted overnight under reflux. After the reaction was completed, the heating was stopped and cooled to room temperature. The reaction solution was poured into water (about 200 mL), and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and further purified by column chromatography (the stationary phase was 350 mesh silica gel, and the eluent was petroleum ether:dichloromethane=15:1 (V / V)), to ob...
Embodiment 3
[0191] Embodiment 3: the synthesis of compound 1-1-D25
[0192] [Synthesis of compound M9]
[0193] The synthetic route of compound M9 is as follows:
[0194]
[0195]Under nitrogen atmosphere, (10-phenylanthracene-9-yl)boronic acid (14.2g, 47.8mmol), anhydrous sodium carbonate (8.4g, 79.6mmol), compound M4 (12.2 g, 39.8mmol), tetrakis(triphenylphosphine palladium) (470.8mg, 4.8mmol) and a mixed solvent of toluene, water and ethanol (100mL, toluene:water:ethanol=5:1:1 (V / V)) . The system was gradually heated to reflux, and reacted overnight under reflux. After the reaction was completed, the heating was stopped and cooled to room temperature. The reaction solution was poured into water (about 200 mL), and extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate, concentrated under reduced pressure, and further purified by column chromatography (the stationary phase was 350 mesh silica gel, and the eluent was petroleum ether:dichlorometh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com