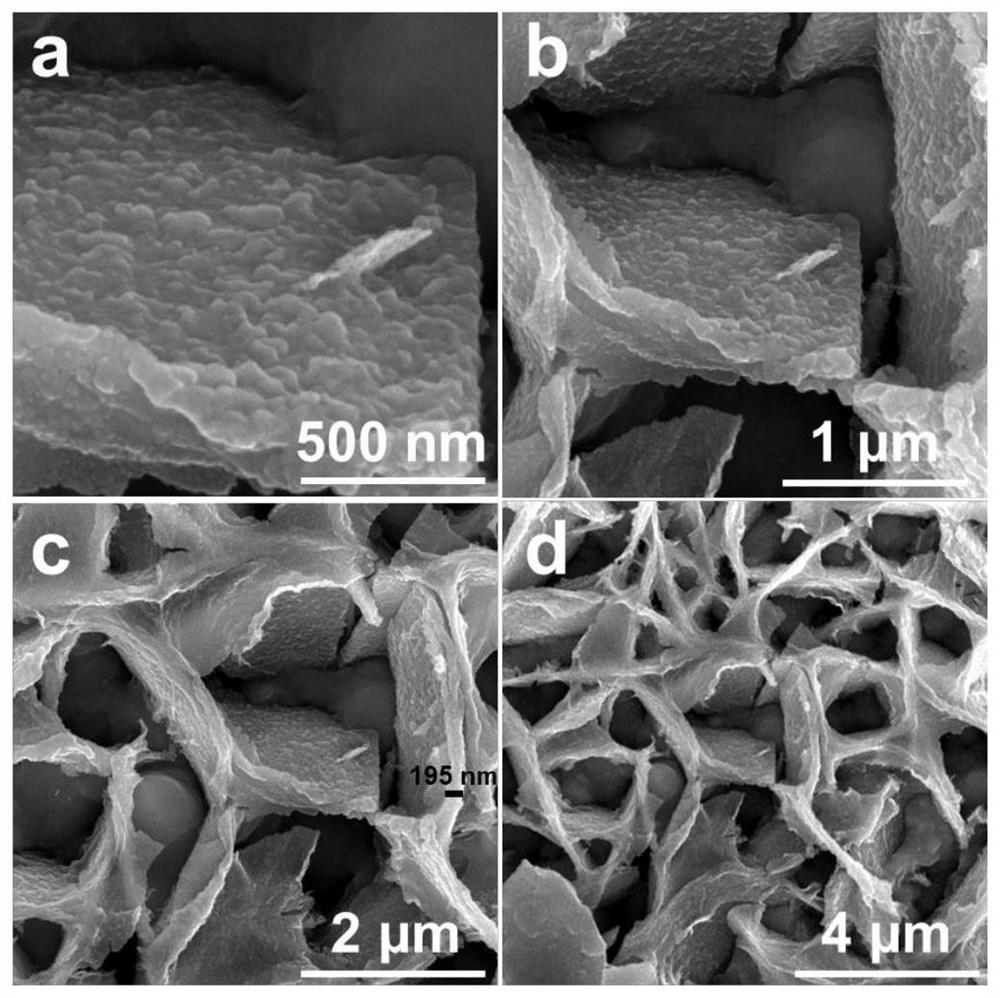
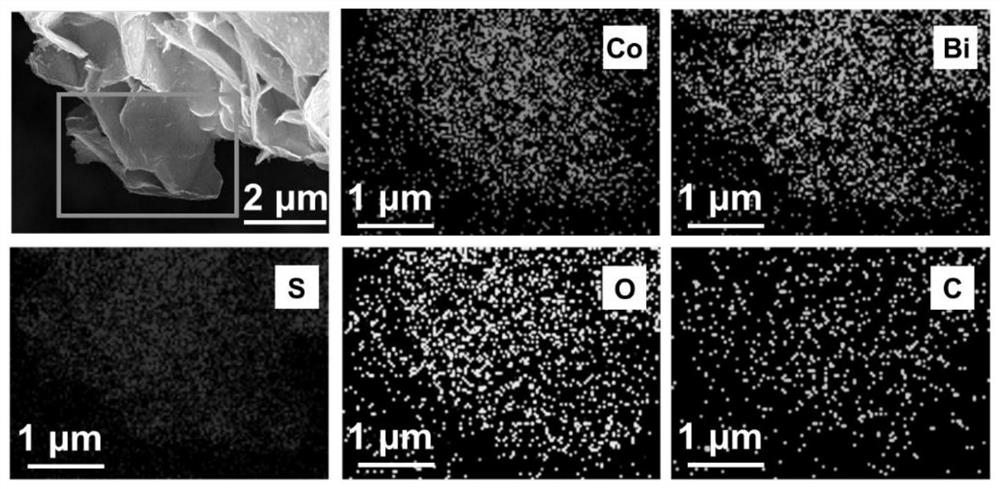
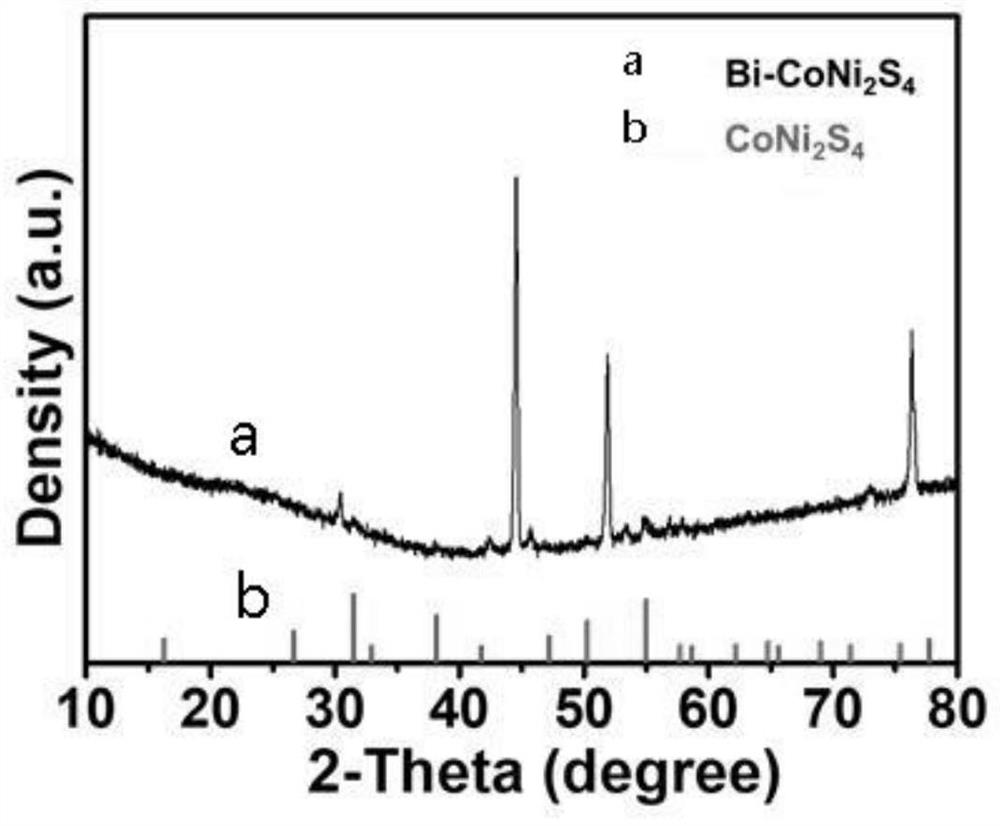
Preparation method of bismuth-doped bimetallic sulfide electrode for electro-catalytic oxidation of urea
An electrocatalytic oxidation and bimetallic technology, applied in the field of nanomaterials, can solve the problem of high cost and achieve the effects of low cost, excellent electrocatalytic performance, and improved hydrogen production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Cut out 1cm*3cm nickel foam, ultrasonically clean it with dilute hydrochloric acid, ethanol, and deionized water for 5 minutes each, and set aside;
[0031] (2) Take a clean beaker, add 25ml deionized water, weigh 0.043g Na 2 BiO 4 2H 2 O, 0.36g Co(NO 3 ) 2 ·6H 2 O and 0.36g Ni(NO 3 ) 2 ·6H 2 O was poured into deionized water, weighed 0.24g 2-methylimidazole and poured into the metal salt mixed solution, ultrasonically dissolved, and poured into a 50 ml reaction kettle; the nickel foam pretreated in step (1) was added to the reaction place in an oven and heat to 120°C, react for 8 hours, and cool down to room temperature naturally after the reaction stops; take out the nickel foam, put it in deionized water for 30 seconds to remove surface deposits, and vacuum dry at 60°C for 12 hours;
[0032] (3) Foamed nickel treated in step (2) in N 2 In the atmosphere, the temperature was raised to 300°C at a rate of 5°C / min, and then the crucible containing 100mg of s...
Embodiment 2
[0045] (1) Cut out 1cm*3cm nickel foam, ultrasonically clean it with dilute hydrochloric acid, ethanol, and deionized water for 5 minutes each, and set aside;
[0046] (2) Take a clean beaker, add 25ml deionized water, weigh 0.043g Na 2 BiO 4 2H 2 O, 0.42g Co(NO 3 ) 2 ·6H 2 O and 0.08g Ni(NO 3 ) 2 ·6H 2 O was poured into deionized water, weighed 0.24g 2-methylimidazole and poured into the metal salt mixed solution, ultrasonically dissolved, and poured into a 50 ml reaction kettle; the nickel foam pretreated in step (1) was added to the reaction place in an oven and heat to 110°C, react for 10 hours, and naturally cool down to room temperature after the reaction stops; take out the nickel foam, put it in deionized water for 30 seconds to remove surface deposits, and vacuum dry at 60°C for 12 hours;
[0047] (3) Foamed nickel treated in step (2) in N 2 In the atmosphere, the temperature was raised to 200 °C at a rate of 10 °C / min, and then the crucible containing 150 mg...
Embodiment 3
[0050] (1) Cut out 1cm*3cm nickel foam, ultrasonically clean it with dilute hydrochloric acid, ethanol, and deionized water for 5 minutes each, and set aside;
[0051] (2) Take a clean beaker, add 25ml deionized water, weigh 0.06g Na 2 BiO 4 2H 2 O, 0.36g Co(NO 3 ) 2 ·6H 2 O and 0.08g Ni(NO 3 ) 2 ·6H 2 O was poured into deionized water, weighed 0.24g 2-methylimidazole and poured into the metal salt mixed solution, ultrasonically dissolved, and poured into a 50 ml reaction kettle; the nickel foam pretreated in step (1) was added to the reaction place in an oven and heat to 180°C, react for 6 hours, and naturally cool down to room temperature after the reaction stops; take out the nickel foam, put it in deionized water for 30 seconds to remove surface deposits, and vacuum dry at 60°C for 12 hours;
[0052] (3) Foamed nickel treated in step (2) in N 2 In the atmosphere, the temperature was raised to 200 °C at a rate of 10 °C / min, and then the crucible containing 120 mg o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Horizontal size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com