Peroxynitrite fluorescent probe, preparation method and application thereof
A peroxynitrite, fluorescent probe technology, applied in fluorescence/phosphorescence, chemical instruments and methods, material analysis by optical means, etc., can solve the problems of insufficient sensitivity and specificity of fluorescent probes, and achieve A stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation process of peroxynitrite fluorescent probe.
[0031]
[0032] Step (1): In a 250mL single-necked round bottom flask, add 150mL of dichloromethane, then add pyridine-4-carbaldehyde (4.5mmol, 0.48g) and 2,4-dimethylpyrrole (9.7mmol, 0.92g) , a drop of trifluoroacetic acid was added as a catalyst, N 2 Protected, stirring rapidly overnight, rotary evaporation to remove the solvent, and then adding chloranil (4.5mmol, 1.1g) to continue the treatment for 30min.
[0033] Step (2): Add 7.5 mL of triethylamine to the product of step (1), and stir for 15 min. Add 7.5mL boron trifluoride diethyl ether at 0°C, and stir at room temperature for 3h. React at 0°C for 2 hours, react at room temperature for 3 hours, wash with saturated sodium hydrogen acid acid solution, separate the organic phase, and dry with anhydrous magnesium sulfate to obtain compound b, whose structural formula is: Step (3): Add compound b (0.09mmol, 0.03g) into a 100mL single-necked round b...
Embodiment 2
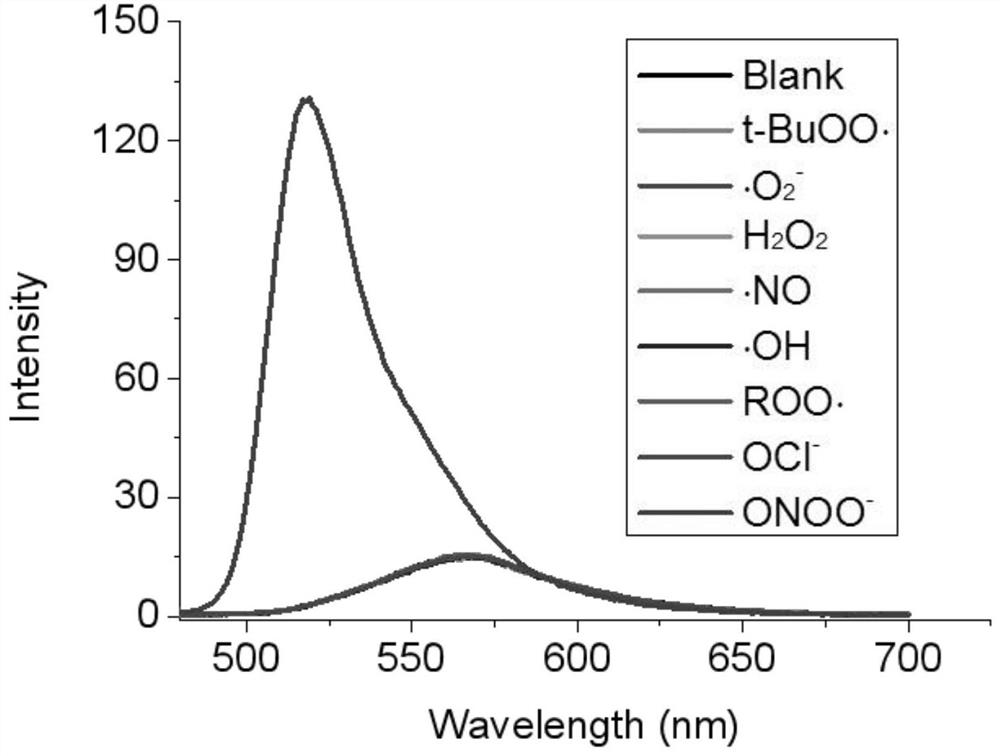
[0035] Embodiment 2: the peroxynitrite fluorescent probe (10 equivalents) gained in embodiment 1 is dissolved in the PBS buffer solution (10%), does not handle, finds that the fluorescence of this probe self is very weak even does not have fluorescence, to OCl was added to the solution - , ROO , OH, NO, H 2 o 2 、 O 2 - , t-BuOO· did not cause any change in fluorescence, but the addition of ONOO - Fluorescence changes were caused after that, indicating that the probe was ONOO - Recognition that exhibits high selectivity, see figure 1 .
[0036] Example 2:
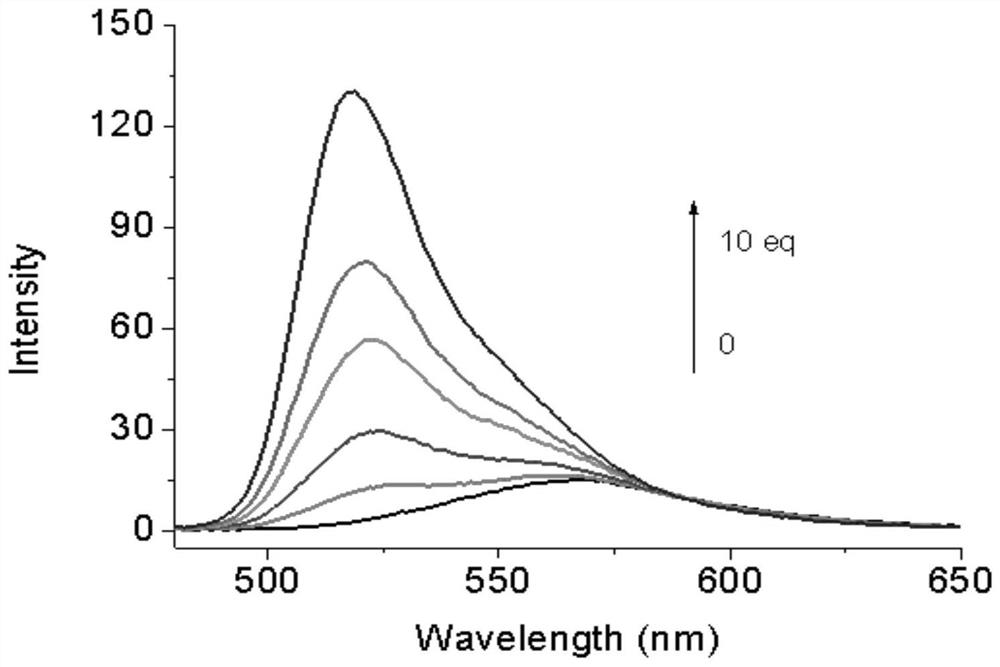
[0037] The peroxynitrite fluorescent probe of the present invention changes with different equivalents of ONOO - Add the change of fluorescence spectrogram, in the present invention, when ONOO - When the equivalent weight of is 10, the fluorescence intensity of the formed compound A is the largest, see figure 2 .
Embodiment 3
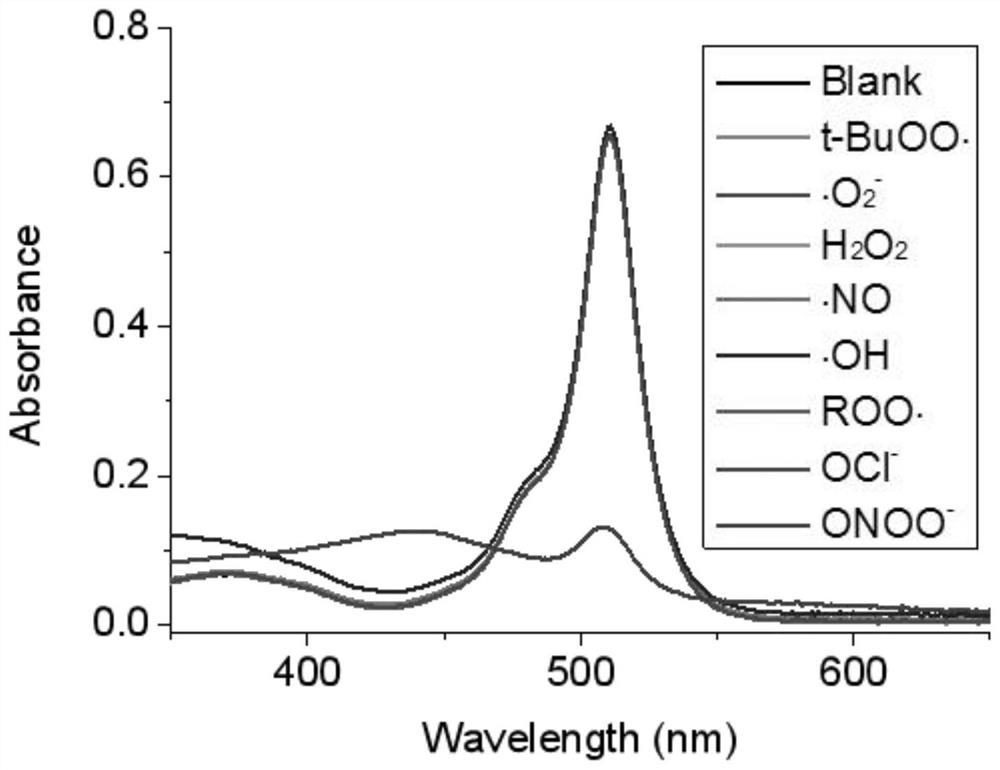
[0039] The fluorescence intensity of the peroxynitrite fluorescent probe itself in the present invention is very little or even no fluorescence, see image 3 . When with ONOO - The compound A formed during the action has a strong fluorescence. In order to prove the formation of the compound A, it was characterized by mass spectrometry, see Figure 4, draw the mechanism of the present invention is that fluorescent probe and ONOO - The effect forms the conclusion of the new compound A.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com