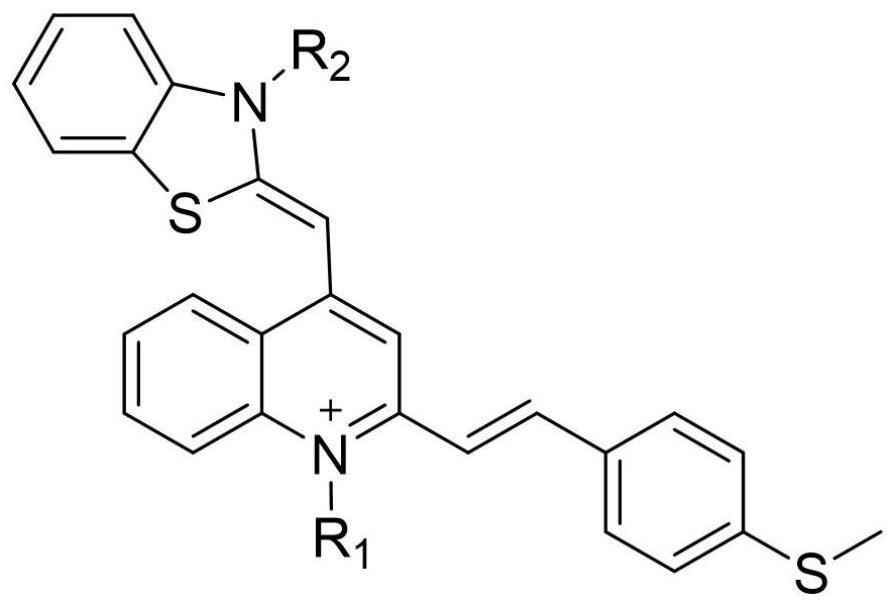
Thiazole orange derivative, preparation and application thereof in mitochondria
A technology of orange derivatives and mitochondria, which is applied in the field of fluorescent probes, can solve the problems that have not been explored clearly, lack of detection methods, and the dynamic folding process of mitochondrial G-quadruplex is not clear, so as to achieve good nucleic acid discrimination effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
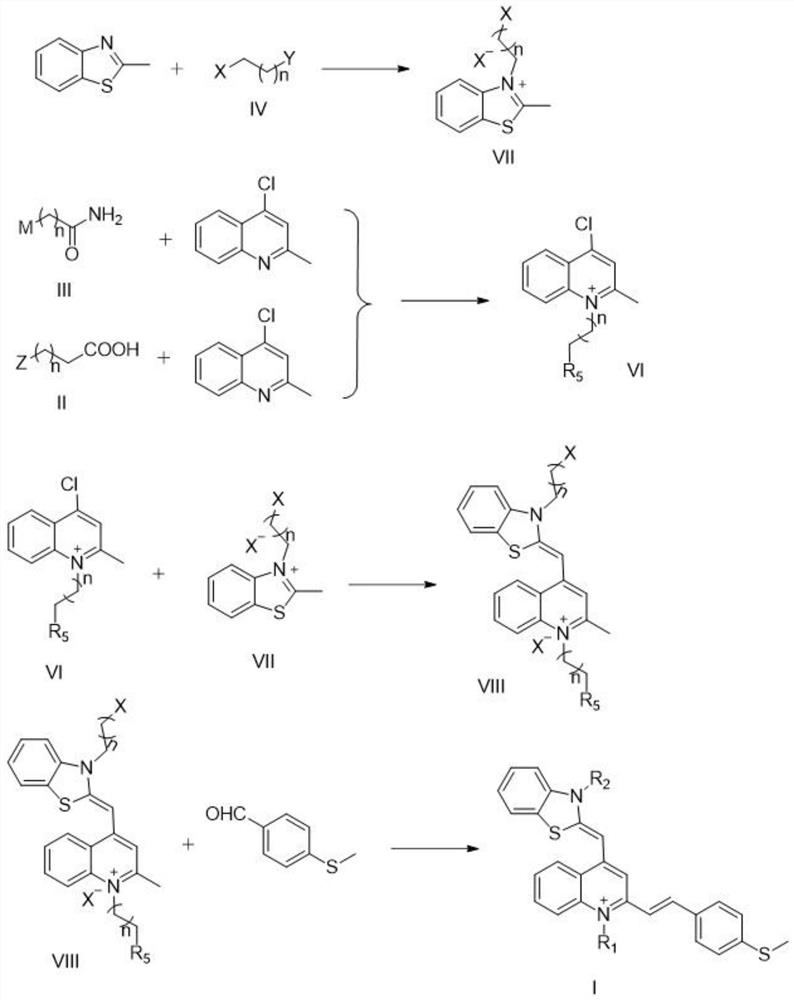
[0064] see figure 2 , figure 2 The synthesis diagram of the preparation method of the thiazole orange derivative provided in the embodiment of the present application, the specific reaction steps of a thiazole orange derivative of the present invention are:
[0065] (1) Add the compound (2mmol) of the formula II structure or the compound (2mmol) of the formula III structure and 4-chloro-2-methylquinoline (2mmol) in 10ml of anhydrous acetonitrile solution, reflux at 100 ℃, react After 24 hours, cool to room temperature, dropwise add 20ml of diethyl ether for recrystallization, a purple-black solid precipitates, collect the solid, and purify to obtain a compound of the product formula VI structure;
[0066] (2) Mix the compound of formula IV structure (0.5mmol) with 2-methylbenzothiazole (1.5mmol), condense and reflux at 85°C, react for 6 hours, after cooling to room temperature, add 10ml of diethyl ether dropwise for recrystallization, and filter Collect the precipitate, th...
Embodiment 1
[0070] The application provides thiazole orange derivatives (I-a), and its synthetic method comprises:
[0071] (1) Add the compound of formula II structure (2mmol) and 4-chloro-2-methylquinoline (2mmol) into 10ml of anhydrous acetonitrile solution, condense and reflux at 100°C, react for 24 hours, cool to room temperature, drop 20ml of diethyl ether was recrystallized, and a purple-black solid was precipitated. The solid was collected and purified to obtain a compound of the product formula VI-a structure;
[0072]
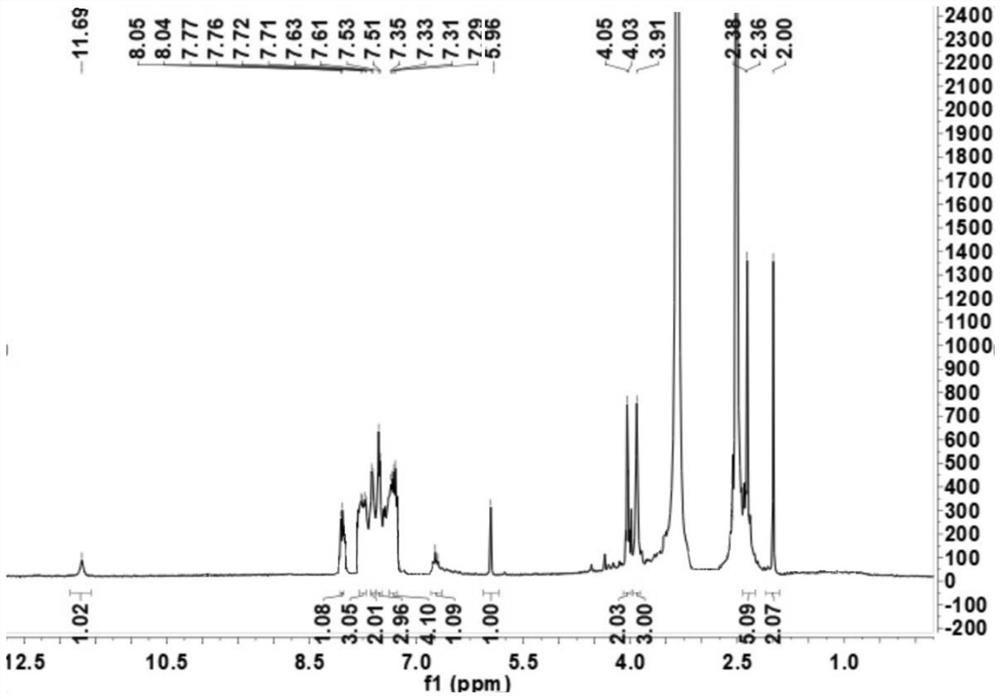
[0073] The compound of formula VI-a structure 1 H NMR result is: 1 H NMR (DMSO, 400Hz): δ12.76(s, 1H), 8.23(m, 3H), 8.16(d, J=8.4Hz, 1H), 8.05–8.01(d, J=8.4Hz, 1H), 7.76–7.73(m,1H),5.07(m,2H),2.89(s,4H),2.80(s,3H); ESI-MS m / z 264.08[M-Br] + ;
[0074] (2) Mix the compound of formula IV structure (0.5mmol) with 2-methylbenzothiazole (1.5mmol), condense and reflux at 85°C, react for 6 hours, after cooling to room temperature, add 10ml of diethyl ether dropwi...
Embodiment 2
[0085] The application provides thiazole orange derivatives (I-b), and its synthetic method comprises:
[0086] (1) Add the compound of formula III structure (2mmol) and 4-chloro-2-methylquinoline (2mmol) into 10ml of anhydrous acetonitrile solution, reflux at 100°C, react for 24 hours, cool to room temperature, drop 20ml of diethyl ether was recrystallized, and a purple-black solid was precipitated. The solid was collected and purified to obtain a compound of the product formula VI-b structure;
[0087]
[0088] The compound of formula VI-b structure 1 H NMR result is: 1 H NMR (DMSO, 400Hz): δ8.59(d, J=7.9Hz, 1H), 8.42(d, J=7.9Hz, 1H), 8.25–8.04(m, 3H), 7.84(s, 2H), 5.57(s,2H),2.99(s,3H); ESI-MS m / z 235.06[M-Br] + ;
[0089] (2) Mix the compound of formula IV-a structure (0.5mmol) with 2-methylbenzothiazole (1.5mmol), condense and reflux at 85°C, react for 6 hours, after cooling to room temperature, add 10ml of diethyl ether dropwise for recrystallization , the precip...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com