Synthesis and application of heteroatom polycyclic aromatic hydrocarbon-containing multi-type organelle fluorescent probe
A technology of polycyclic aromatic hydrocarbons and fluorescent probes, which is applied in the direction of fluorescence/phosphorescence, instruments, and luminescent materials, etc. It can solve the problems of cumbersome synthesis routes, low yields, low atom/step economy, etc., and achieve good photostability , The synthesis route is simple, and the effect of biocompatibility is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
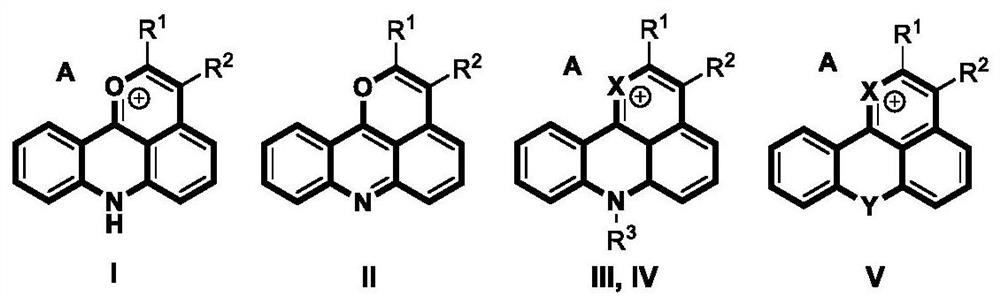
[0052] Example 1: Synthesis of 2,3-diphenylpyrano[4,3,2-kl]acridinium 7-ium hexafluoroantimonate (I-a)
[0053] (1) Add acridone (39.1mg, 0.20mmol), toluene (71.2mg, 0.40mmol), [Cp*RhCl 2 ] 2 (3.1mg, 5μmol, 2.5mol%), AgOAc (66.8mg, 0.40mmol), AgSbF 6 (13.7mg, 40μmol, 20mol%), NaSbF 6 (51.7mg, 0.20mmol) and tetrahydrofuran (1.0mL) were added to the reaction tube, stirred evenly, heated to 80°C, and reacted for 10 hours;
[0054] (2) After the reaction is completed, cool the reaction tube to room temperature, add 10 mL of dichloromethane to dilute the reaction system, then filter through diatomaceous earth and wash with 10-20 mL of dichloromethane, combine the filtrates, remove the solvent under reduced pressure, and the remaining The product was separated and purified by silica gel column chromatography (dichloromethane / ethyl acetate=15:1, v / v), and after vacuum drying, the target product 2,3-diphenylpyran[4,3,2 -kl] Acridinium 7-ium hexafluoroantimonate (I-a) 81.3 mg, yiel...
Embodiment 2
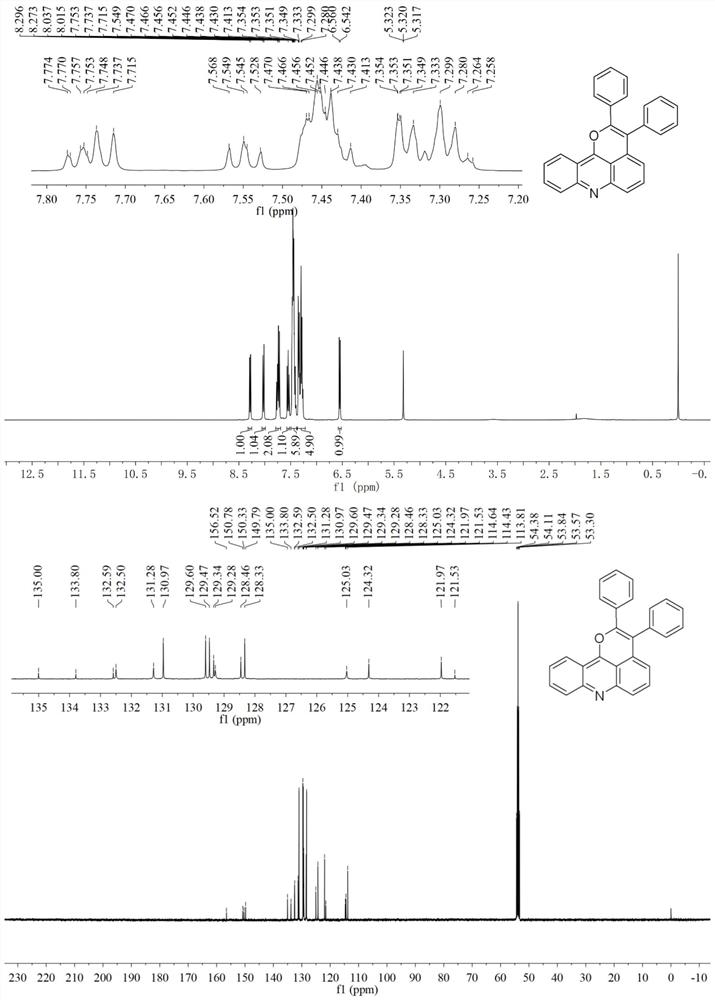
[0055] Embodiment 2: Synthesis of 2,3-diphenylpyrano[4,3,2-kl]acridine (II-a)
[0056] (1) Add acridone (39.1mg, 0.20mmol), toluene (71.2mg, 0.40mmol), [Cp*RhCl 2 ] 2 (3.1mg, 5μmol, 2.5mol%), AgOAc (66.8mg, 0.40mmol), AgSbF 6 (13.7mg, 40μmol, 20mol%), NaSbF 6 (51.7mg, 0.20mmol) and tetrahydrofuran (1.0mL) were added to the reaction tube, stirred evenly, heated to 80°C, and reacted for 10 hours;
[0057] (2) After the reaction is completed, cool the reaction tube to room temperature, add 10 mL of dichloromethane to dilute the reaction system, then filter through diatomaceous earth and wash with 10-20 mL of dichloromethane, combine the filtrates, remove the solvent under reduced pressure, and the remaining The product was separated and purified by neutral aluminum oxide column chromatography (dichloromethane / ethyl acetate=10:1, v / v), and after vacuum drying, the target product 2,3-diphenylpyran[ 4,3,2-kl] acridine (II-a) 55.7 mg, yield 75%. 1 H NMR (400MHz, CD 2 Cl 2 ):δ=...
Embodiment 3
[0058] Example 3: Synthesis of 2,3-diphenyl-7-methyl-7H-pyrano[4,3,2-kl]acridin-1-ium hexafluoroantimonate (III-a)
[0059] (1) Mix N-methylacridone (41.8mg, 0.20mmol), toluene (71.2mg, 0.40mmol), [Cp*RhCl 2 ] 2 (3.1mg, 5μmol, 2.5mol%), AgOAc (66.8mg, 0.40mmol), AgSbF 6 (13.7mg, 40μmol, 20mol%), NaSbF 6 (51.7mg, 0.20mmol) and tetrahydrofuran (1.0mL) were added to the reaction tube, stirred evenly, heated to 80°C, and reacted for 10 hours;
[0060] (2) After the reaction is completed, cool the reaction tube to room temperature, add 10 mL of dichloromethane to dilute the reaction system, then filter through diatomaceous earth and wash with 10-20 mL of dichloromethane, combine the filtrates, remove the solvent under reduced pressure, and the remaining The product was separated and purified by silica gel column chromatography (dichloromethane / ethyl acetate=20:1, v / v), and after vacuum drying, the target product 2,3-diphenyl-7-methyl-7H- Pyran[4,3,2-kl]acridin-1-ium hexafluoroa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com