Penehyclidine hydrochloride impurity and preparation method thereof
A technology for penehyclidine and impurities, applied in the field of penehyclidine hydrochloride impurity and preparation thereof, can solve problems such as no literature report in compound preparation method, and achieve the effects of low cost, simple and convenient operation, and strong practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
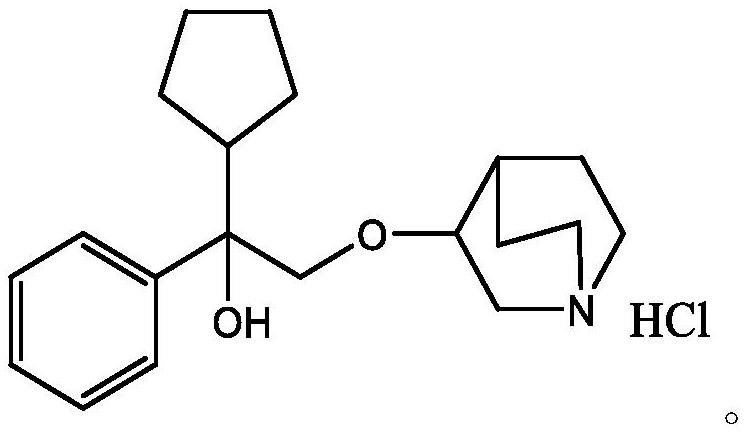
[0037] Embodiment 1 Penhyclidine base preparation:
[0038] Add 20g of penehyclidine hydrochloride, 50ml of 10% NaOH solution, 100ml of ethyl acetate into a 250ml beaker, stir and clarify, let stand to separate the liquids, extract the aqueous phase twice with ethyl acetate (200ml / time), combine the organic phases, and saturate Washed with brine, dried over anhydrous magnesium sulfate, and concentrated to dryness to obtain a colorless oil.
Embodiment 2
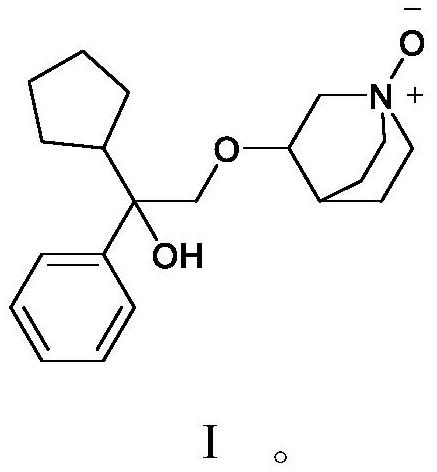
[0039] Embodiment 2: the preparation method of compound shown in formula I
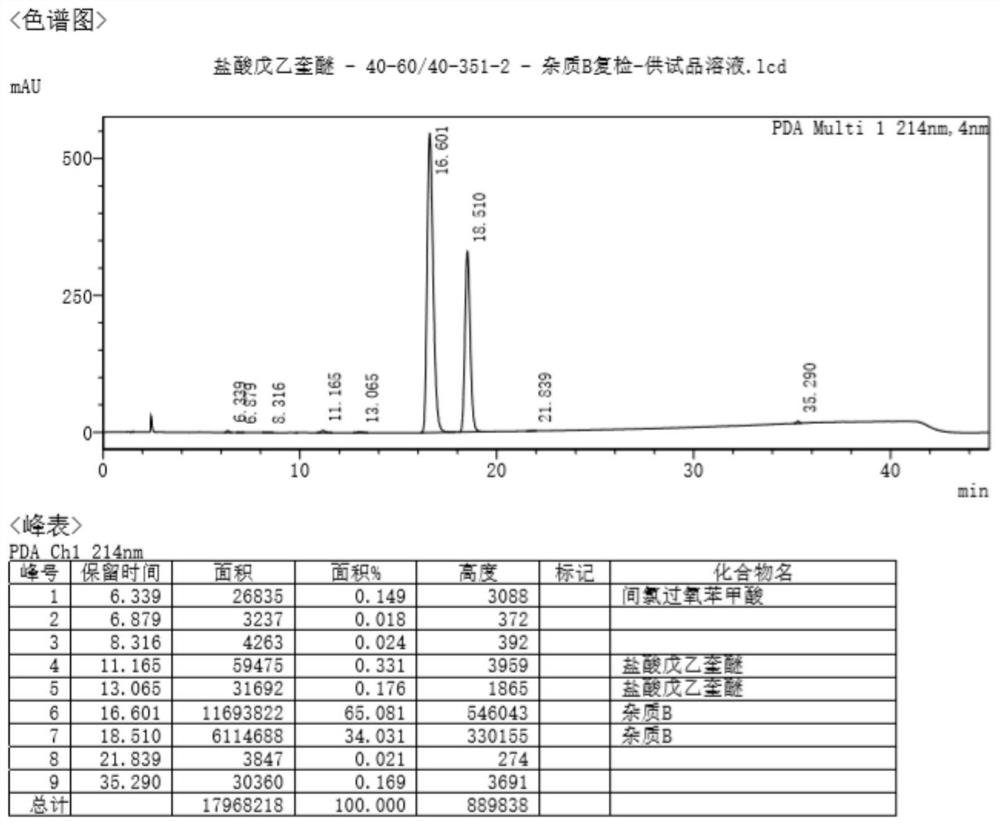
[0040] Add all the oils prepared in Example 1 and 300ml THF to the 500 reaction flask, stir and dissolve, add 15.7g m-chloroperoxybenzoic acid in batches, after the addition is complete, stir at room temperature for 36 hours.
[0041] Add 200ml of saturated sodium carbonate solution to the reaction solution, adjust the pH to 8-9, let stand to separate layers, extract the water phase with tetrahydrofuran twice (200ml / time), combine the organic phases, dry over anhydrous magnesium sulfate, and concentrate to dryness. 15.3 g of yellow oil was obtained.
[0042] The crude product was dissolved in 100ml of tetrahydrofuran, 15.3g of neutral aluminum oxide (200-300 mesh) was added, concentrated under reduced pressure to dryness, and the sample was used for later use. Another 153g of neutral aluminum oxide (200-300 mesh) was added to 2L of ethyl acetate, stirred evenly, added to a glass chromatography column...
Embodiment 3
[0045] The preparation method of compound shown in embodiment 3 formula I
[0046] (1) Add all the oils prepared in Example 1 and 300ml THF to the 500 reaction flask, stir and dissolve, add 15.7g m-chloroperoxybenzoic acid in batches, after adding, stir at room temperature for 28 hours.
[0047](2) Add 200ml of saturated sodium carbonate solution to the reaction solution, adjust the pH to 8-9, let the layers stand, and extract the aqueous phase twice with THF (200ml / time), combine the organic phases, and dry over anhydrous magnesium sulfate , concentrated to dryness to obtain 16.0 g of yellow oil.
[0048] (3) Dissolve the crude product in 100 ml of tetrahydrofuran, add 32 g of neutral aluminum oxide (200-300 mesh), concentrate to dryness under reduced pressure, and prepare the sample for later use. In addition, add 320g of neutral aluminum oxide (200-300 mesh) into 2L of ethyl acetate, stir evenly, add it to a glass chromatography column for sedimentation, pressurize the col...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com