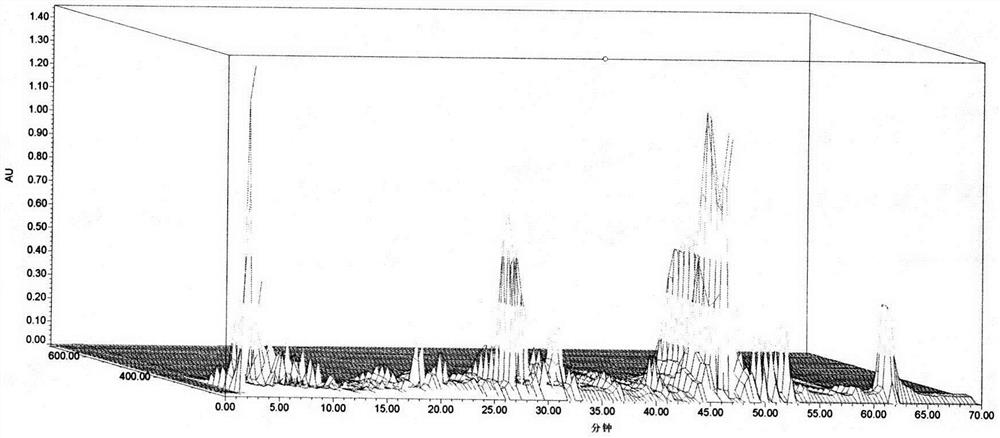
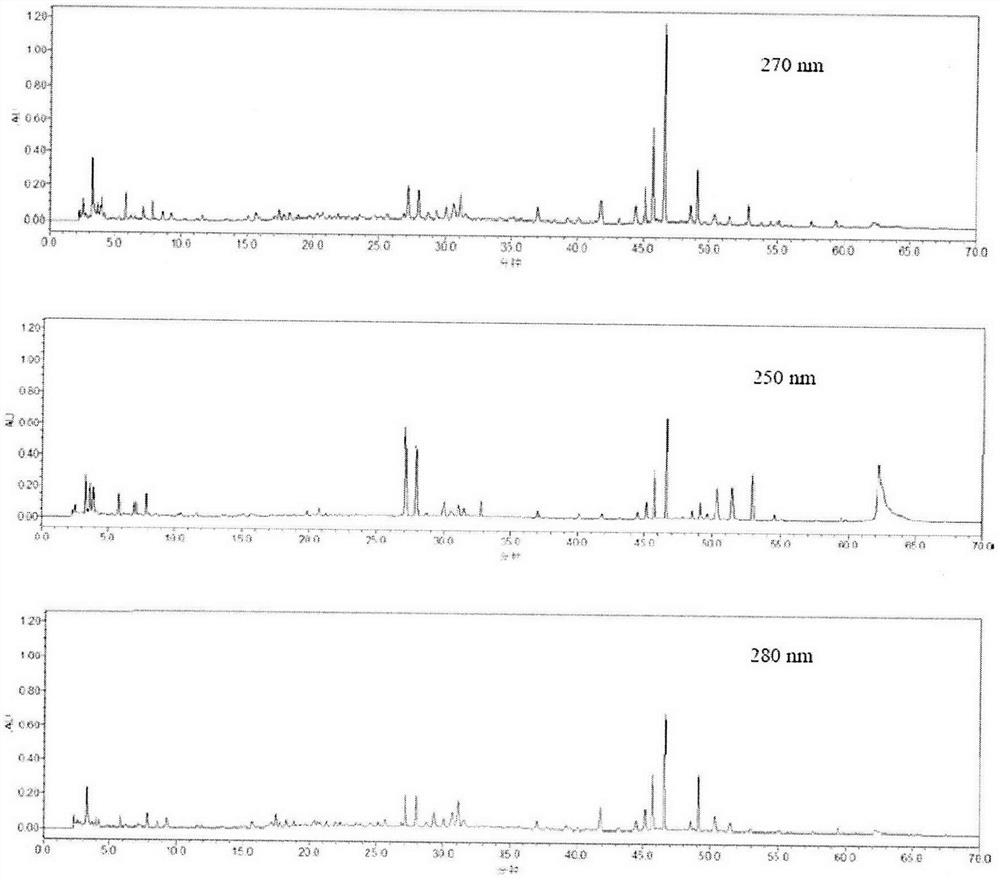
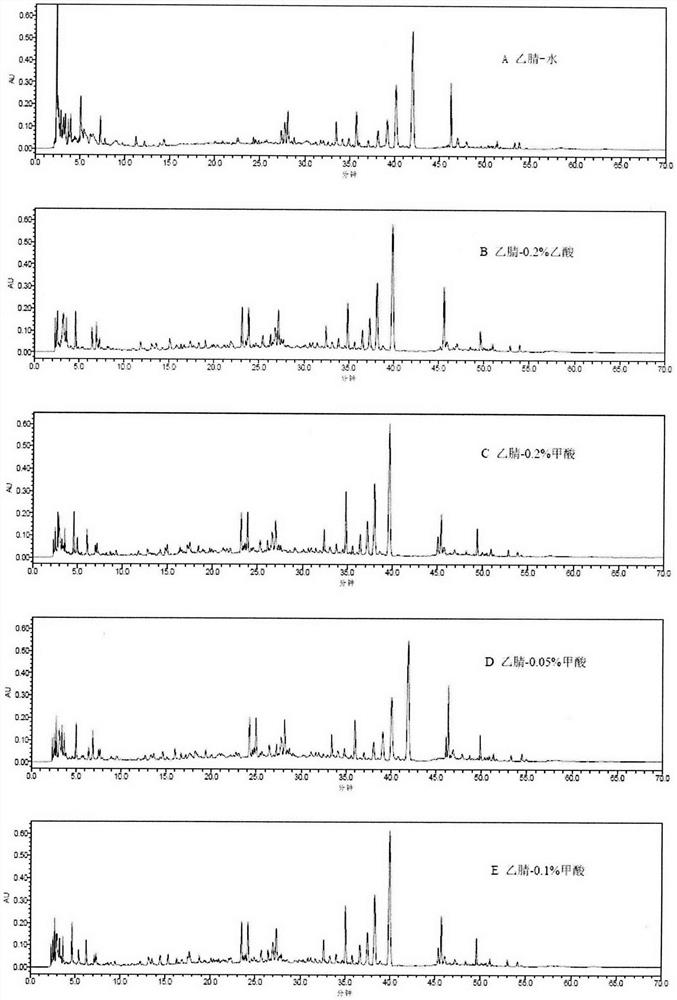
Method for detecting fingerprint spectrum of Shengbai Koufuye
A technology of Shengbai oral liquid and standard fingerprint, applied in the field of fingerprint detection of Shengbai oral liquid, can solve problems such as insufficient solution to multi-component complex system of traditional Chinese medicine preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] The preparation of embodiment 1 Shengbai oral liquid
[0107] Step (1) consists of 240g of epimedium, 120g of psoraleae, 80g of aconite (made), 240g of medlar, 240g of astragalus, 240g of Caulis Spatholobus, 240g of madder, 120g of angelica, 240g of reed root, 120g of Radix Ophiopogon japonicus and licorice 120g spare;
[0108] Step (2) First put in the prescription amount of Shiwei decoction pieces (excluding Epimedium), add water, heat and decoct until boiling, then add the prescription amount of Epimedium, continue heating and boiling until boiling, extract for 1 hour, filter, Obtain one extraction filtrate, add water to the medicinal dregs and extract once again, filter to obtain the second extraction filtrate, combine the two filtrates, and concentrate the filtrate to extract I with a relative density of 1.24-1.27 (25 ± 5°C);
[0109] Step (3) slowly add 90% ethanol according to extract I: ethanol=1:1~1.2 in extract I, stir while adding, extract extract is dispers...
Embodiment 2
[0111] The preparation of embodiment 2 Shengbai oral liquid
[0112] Step (1) Epimedium 200g, psoraleae 100g, aconite (manufactured) 60g, medlar 200g, astragalus 200g, Caulis Spatholobus 200g, madder 200g, angelica 100g, reed root 200g, Ophiopogon japonicus 100g and licorice 100g for later use ;
[0113] Step (2) First put in the prescription amount of Shiwei decoction pieces (excluding Epimedium), add water, heat and decoct until boiling, then add the prescription amount of Epimedium, continue heating and boiling until boiling, extract for 1 hour, filter, Obtain one extraction filtrate, add water to the medicinal dregs and extract once again, filter to obtain the second extraction filtrate, combine the two filtrates, and concentrate the filtrate to extract I with a relative density of 1.24-1.27 (25 ± 5°C);
[0114] Step (3) slowly add 90% ethanol according to extract I: ethanol=1:1~1.2 in extract I, stir while adding, extract extract is dispersed evenly, then add 90% ethanol...
Embodiment 3
[0116] The preparation of embodiment 3 Shengbai oral liquid
[0117] Step (1) Take Epimedium 300g, Psoralen 160g, Aconite (manufactured) 120g, Lycium barbarum 300g, Astragalus 300g, Caulis Spatholobus 300g, Rubia 300g, Angelica 160g, Reed root 300g, Ophiopogon japonicus 160g and Licorice 160g for later use ;
[0118] Step (2) First put in the prescription amount of Shiwei decoction pieces (excluding Epimedium), add water, heat and decoct until boiling, then add the prescription amount of Epimedium, continue heating and boiling until boiling, extract for 1 hour, filter, Obtain one extraction filtrate, add water to the medicinal dregs and extract once again, filter to obtain the second extraction filtrate, combine the two filtrates, and concentrate the filtrate to extract I with a relative density of 1.24-1.27 (25 ± 5°C);
[0119] Step (3) slowly add 90% ethanol according to extract I: ethanol=1:1~1.2 in extract I, stir while adding, extract extract is dispersed evenly, then ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com