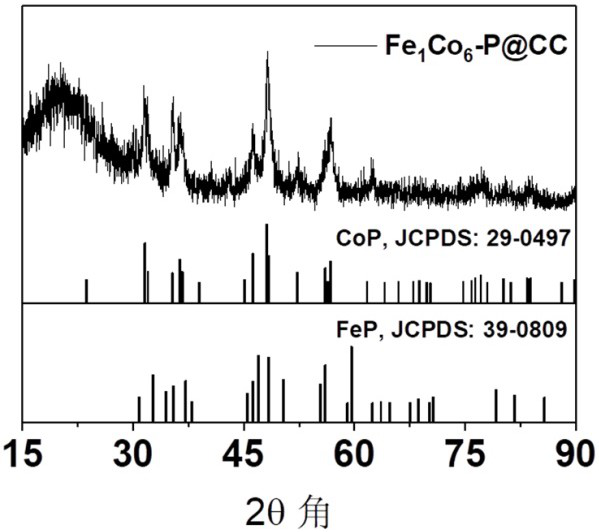
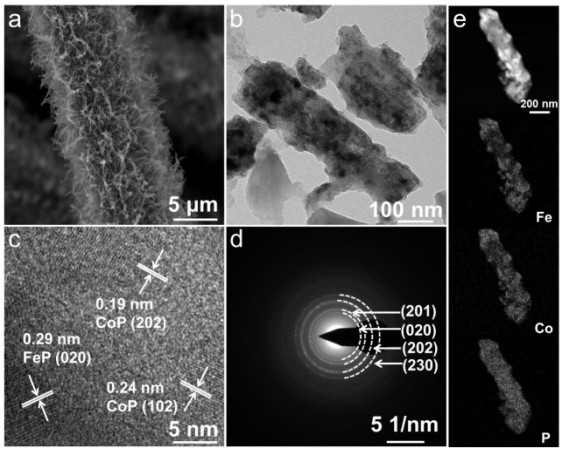
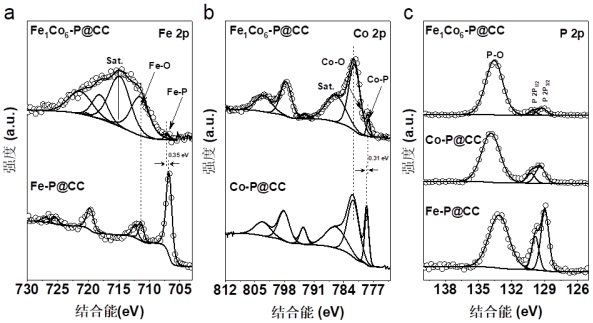
a fe 1 co 6 -p@cc electrocatalyst and preparation method thereof
A fe1co6-p, electrocatalyst technology, applied in catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of unfavorable electrolysis of water for hydrogen production and oxygen production, high cost, scarce price and other problems, Achieve the effects of excellent electrocatalytic water splitting performance, long service life, and simple and easy preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0027] 3. Example 2: Preparation of Fe-P@CC composite material.
[0028] Step (1) Cut the carbon cloth into 1.5×4 cm 2 size, and then ultrasonically cleaned in 1 mol / L hydrochloric acid solution, deionized water and ethanol for 10 minutes, washed in a cycle three times, and dried naturally for later use.
[0029] Step (2) Prepare ferric nitrate nonahydrate mixed solution: weigh 1.980g ferric nitrate nonahydrate, 0.119g ammonium fluoride and 0.481g urea, dissolve them in 70 mL deionized water and 5 mL ethanol, and ultrasonicate for 3 minutes to form a The mixed solution of ferric nitrate nonahydrate is ready for use.
[0030] Step (3) Hydrothermal reaction: Transfer the prepared ferric nitrate nonahydrate mixed solution to a 90 mL polytetrafluoroethylene-lined stainless steel reactor, then place the treated carbon in the mixed solution, and place the reactor Put it in an oven and heat it to 140°C to react for 10 hours. After the reaction, take out the carbon cloth and rinse i...
Embodiment 3
[0032] 4. Example 3: Preparation of Co-P@CC composite material.
[0033] Step (1) process the carbon cloth: cut the carbon cloth into 1.5×4 cm 2 size, and then ultrasonically cleaned in 1 mol / L hydrochloric acid solution, deionized water and ethanol for 10 minutes, washed in a cycle three times, and dried naturally for later use.
[0034] Step (2) Prepare the mixed solution of cobalt nitrate hexahydrate: weigh 1.426g cobalt nitrate hexahydrate, 0.119g ammonium fluoride and 0.481g urea, dissolve them in 70 mL deionized water and 5 mL ethanol, and ultrasonicate for 3 minutes to form a The mixed solution of cobalt nitrate hexahydrate is ready for use.
[0035] Step (3) Transfer the prepared cobalt nitrate hexahydrate mixed solution to a 90 mL polytetrafluoroethylene-lined stainless steel reactor, then arrange the treated carbon in the mixed solution, and place the reactor in an oven Heat to 140 °C for 10 hours. After the reaction, take out the carbon cloth and wash it three tim...
Embodiment 5
[0042] Sixth, embodiment 5: preparation Fe 1 co 8 -P@CC composites.
[0043] Step (1) process the carbon cloth: cut the carbon cloth into 1.5×4 cm 2 size, and then ultrasonically cleaned in 1 mol / L hydrochloric acid solution, deionized water and ethanol for 10 minutes, washed in a cycle three times, and dried naturally for later use.
[0044] Step (2) Prepare iron-cobalt mixed solution (the total amount of metal salt is 4.9 mmol, the molar ratio of Fe and Co is 1:8): weigh 1.268g cobalt nitrate hexahydrate, 0.220g ferric nitrate nonahydrate, 0.119g fluorine Ammonium chloride and 0.481 g of urea were dissolved in 70 mL of deionized water and 5 mL of ethanol, and ultrasonicated for 3 minutes to form an iron-cobalt mixed solution for later use.
[0045] Step (3) Hydrothermal reaction: Transfer the prepared iron-cobalt mixed solution to a 90 mL polytetrafluoroethylene-lined stainless steel reactor, then arrange the treated carbon in the mixed solution, and place the reactor on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com