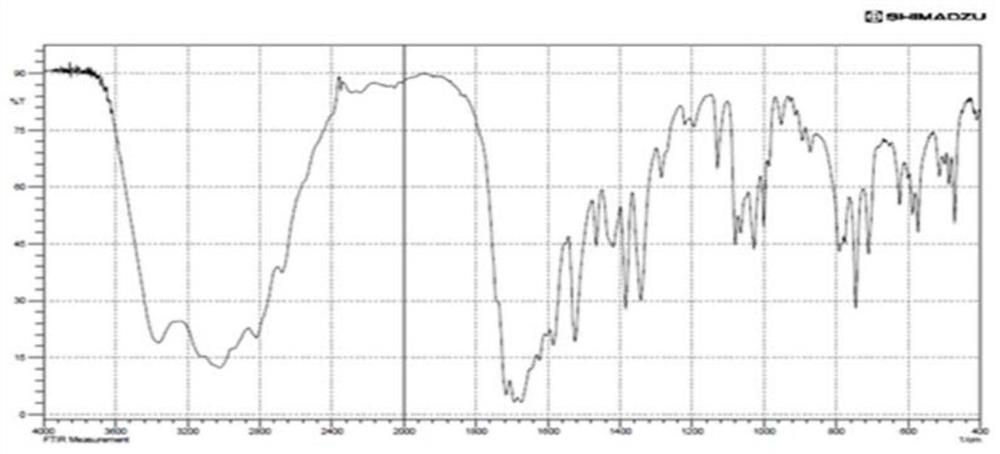
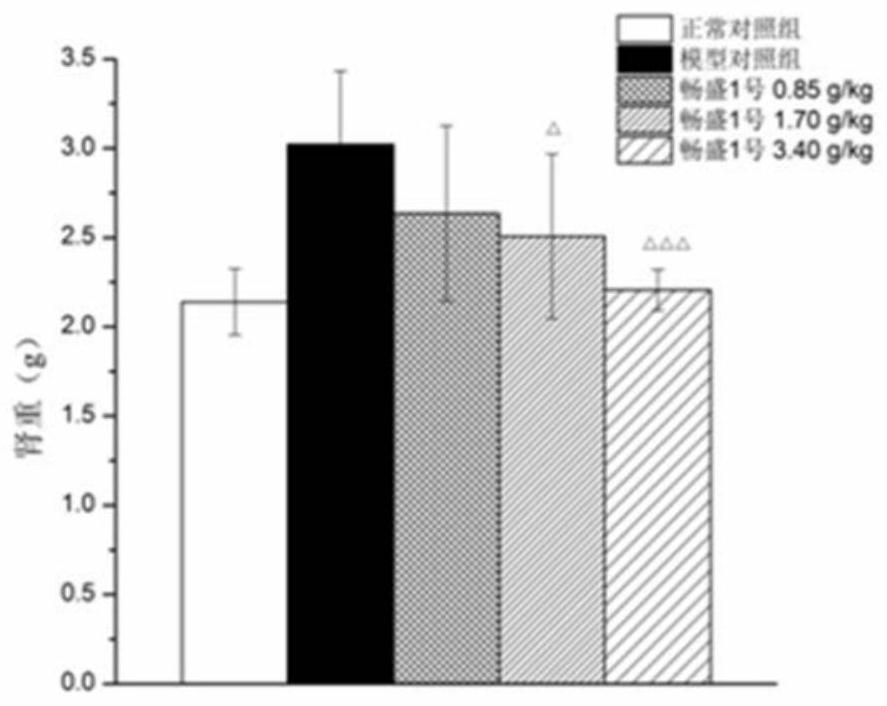
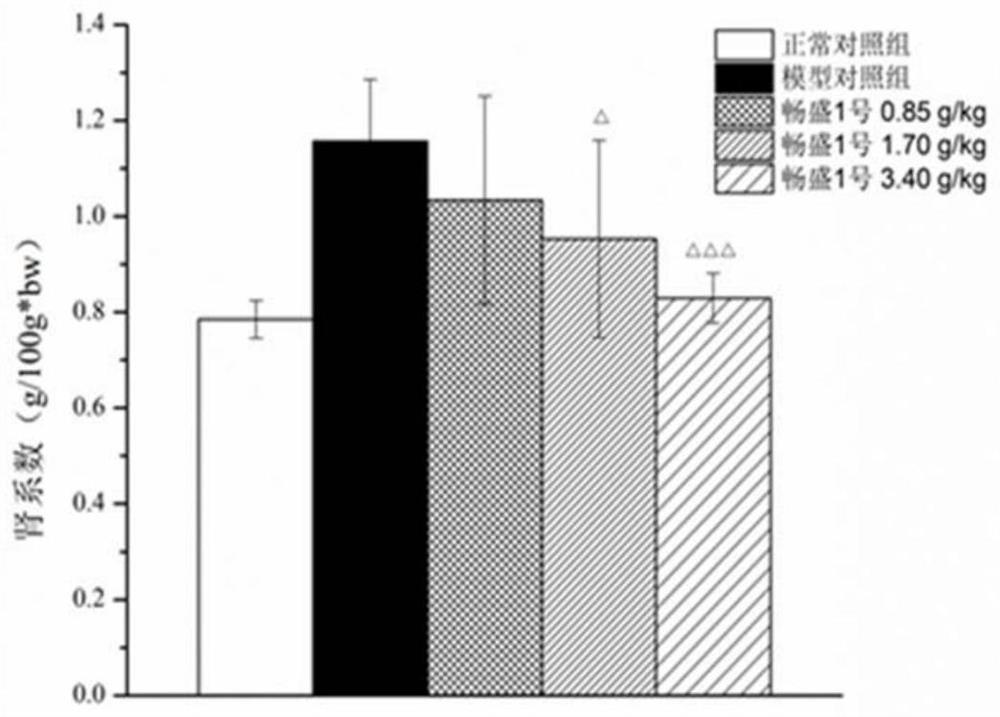
Application of tri(hydroxymethyl)aminomethane hydrochloride solution in medicine for treating hyperuricemia
A tris, hyperuricemia technology, applied in the field of pharmaceutical applications, can solve the problem of no tris hydrochloride solution, etc., to achieve the effect of increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Tris and uric acid combined reaction to form "uric acid·TRIs conjugate" and preliminary research experiments on "uric acid·TRIs conjugate":
[0146] 1. Synthesis
[0147] Weigh 300 mg of uric acid, add it to 100 ml of 0.6mol / L (PH7.4) tris hydrochloride solution, stir for 10 minutes, the uric acid is completely dissolved, the solution becomes clear, continue to stir, and then a milky flocculent precipitate is formed , Vacuum filtration and drying to obtain 480 mg of white blocky uric acid·trishydroxymethylaminomethane conjugate.
[0148] 2. Solubility
[0149] ① Take 56mg of white blocky uric acid·trishydroxymethylaminomethane conjugate and add it to 100ml of water, stir for 1 hour, the white block is completely dissolved, the solution is clear and no longer crystallized. However, continuing to add uric acid-trishydroxymethylaminomethane conjugates in this solution will make the solution supersaturated and make part of the conjugates insoluble.
[0150] It shows that...
Embodiment 2
[0157] Imitating the concentration of the drug in the blood after the drug is used in the human body, the test of dissolving uric acid crystals in water
[0158] Test material: 1, uric acid (crystal), 2, Tris hydrochloride solution (PH7.4 ± 0.5) 0.3mol / L or 0.6mol / L solution (new medicine of the present invention), 3, water for injection.
[0159] Experimental Design: In Vitro Simulation Test
[0160] Assuming a 60 kg heavy hyperuric acid patient, its total blood 4500ml, 7mg uric acid is dissolved in 100ml blood; 3mg uric acid crystals are arranged, now use new drug trishydrochloride hydrochloride solution of the present invention (PH7.4 ± 0.5) 0.3mol / L or 0.6mol / L solution treatment, the dosage is 0.5ml / kg-3ml / kg for one administration.
[0161] 1. Assuming that the total blood volume of a 60 kg patient is 4500ml, 7mg of uric acid is dissolved in 100ml of blood;
[0162] Corresponding test: Take 10mg of uric acid + 100ml of water + 0.66ml, 0.3mol / L liquid medicine, put it i...
Embodiment 3
[0181] Dissolution test of sodium urate crystal in medicinal liquid of the present invention
[0182] 1. Take 0.3 mol / L tris hydrochloride solution (PH7.4±0.5), 35 ml, add 50 mg of sodium urate, put it in a shaker at 36.5 °C for 60 rpm, keep warm and shake for 4 hours, solid particles Completely dissolved, the solution was clear.
[0183] 2. Take 35 ml of purified water, add 50 mg of sodium urate, put it in a shaker at 36.5°C for 60 rpm, keep it warm and shake for 4 hours, most of the solid particles are not dissolved, and the solution is very turbid.
[0184] Test result: illustrate that medicinal liquid of the present invention can dissolve sodium urate crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com