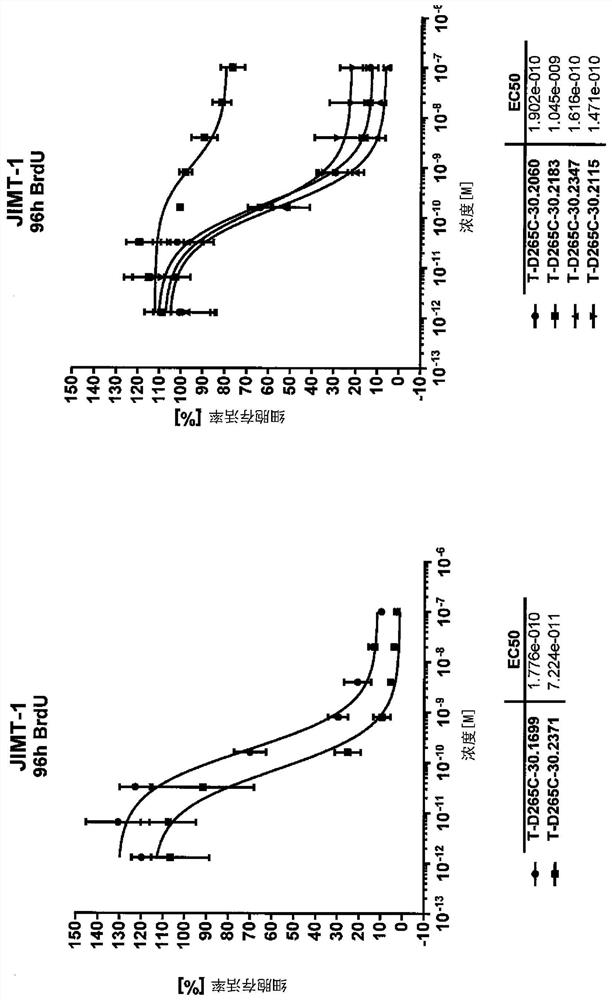
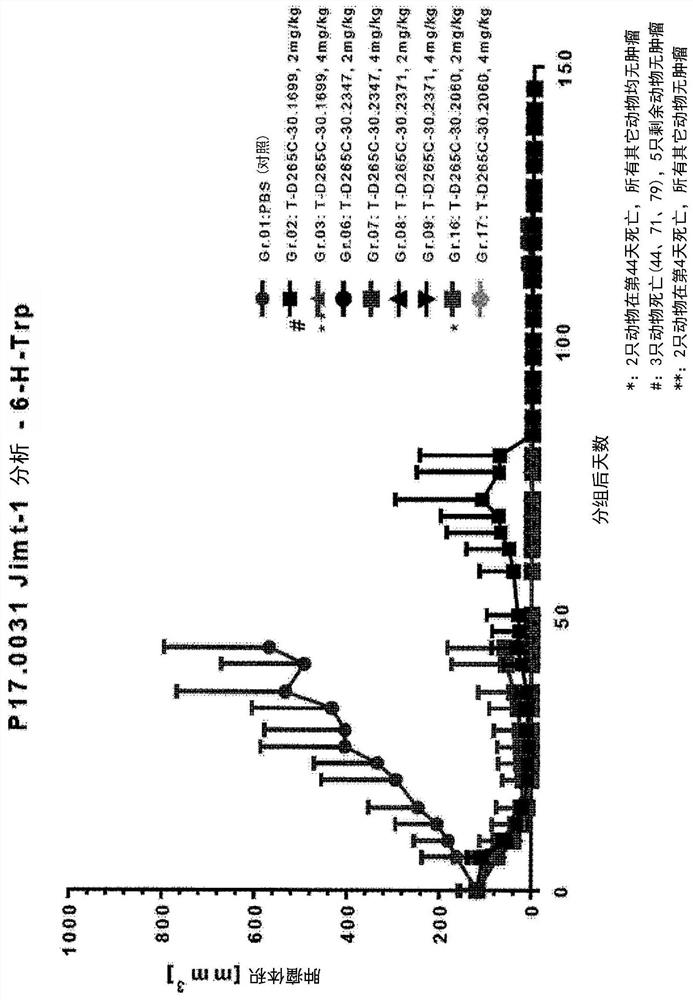
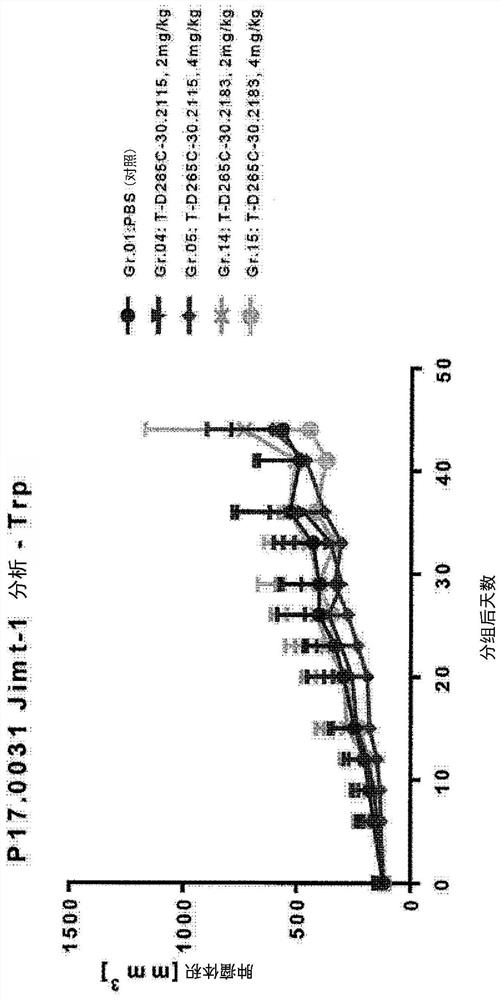
Targeted amatoxin conjugate for the treatment of solid tumors
A technology of amanita toxin and conjugate, which is applied in the directions of antitumor drugs, drug combinations, medical preparations of inactive ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0065] According to yet another embodiment, the linker comprises a motif selected from the group consisting of:
[0066] ·Val Ala
[0067] ·Val Cit
[0068] ·Val Lys
[0069] ·Val Arg
[0070] ·Phe Lys Gly Pro Leu Gly
[0071] ·Ala Ala Pro Val
[0072] · β-glucuronide
[0073] · β-galactoside.
[0074] According to yet another embodiment, the reactive group Y in the linker is at least one selected from the following:
[0075] ·Maleimide
[0076] 3,4-Diphenylsulfanyl-maleimide
[0077] 4,5-bis(2-pyridyl-sulfanyl)-1,2-dihydropyridazine-3,6-dione
[0078] ·DBCO
[0079] ·DIBO
[0080] ·HIPS
[0081] 2-(4-Hydroxyphenyl)-5-(methylsulfonyl)-1,3,4-oxadiazole
[0082] ·APN
[0083] ·Succinimide
[0084] ·Succinimidyl carbamate
[0085] ·Bromoacetamide
[0086] ·Iodoacetamide
[0087] According to yet another embodiment, the linker comprises a p-aminobenzene ValAla maleimidopropyl motif.
[0088]
[0089] According to yet another embodiment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com