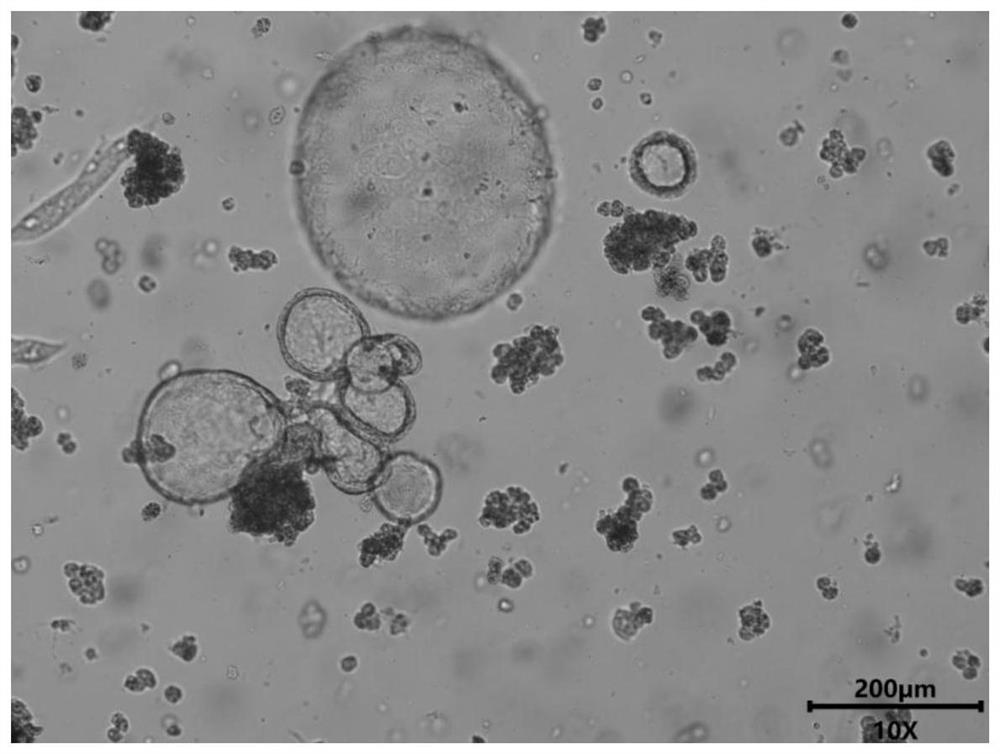
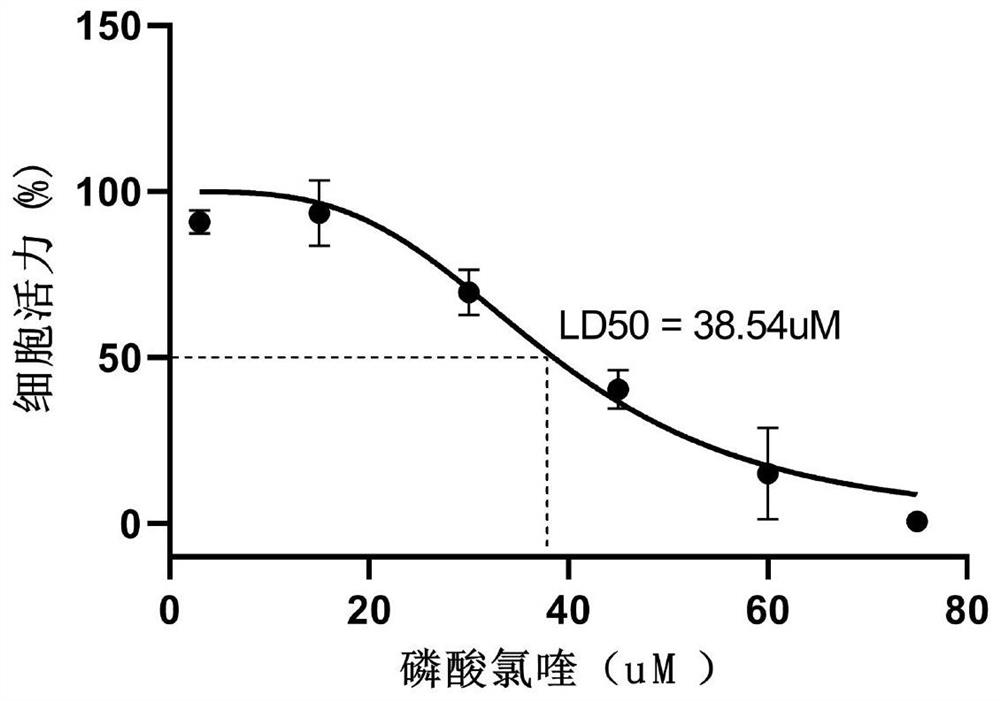
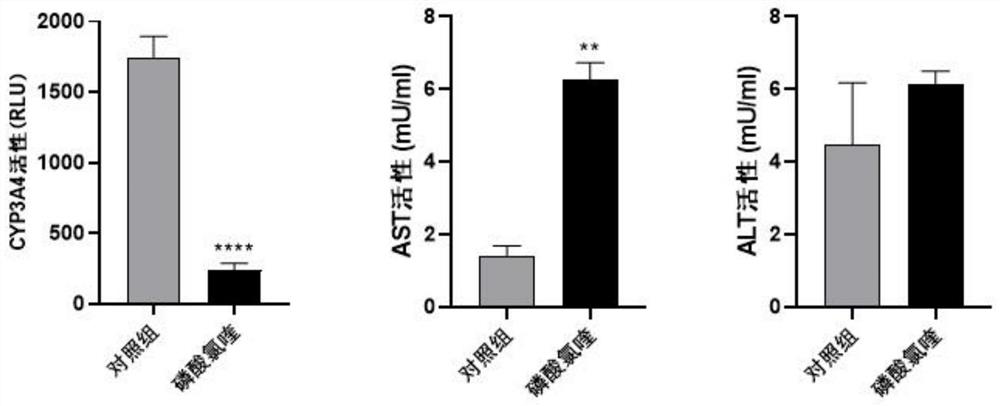
Liver organoid model-based drug hepatotoxicity evaluation method
An evaluation method and organoid technology, applied in the field of biomedicine, can solve the problems of limited practicability and long-term culture capacity, and achieve the effects of reducing the cost of new drug development, easy operation, and reducing labor costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This embodiment provides a method for evaluating hepatotoxicity of chloroquine phosphate based on a liver organoid model, comprising the following steps:
[0040] 1. Establishment of mouse liver organoid model:
[0041] 1) Sample cleaning: the mouse liver tissue was washed several times with normal saline containing 1% double antibody, and the obvious fat tissue was removed.
[0042] 2) Sample cutting: Cut the tissue into small pieces of about 0.5mm^3.
[0043] 3) Tissue digestion: ①Disperse the sheared tissue in normal saline, centrifuge to remove the supernatant, add collagenase, dispase and DNaseI to the precipitate, shake and digest at 37°C for 30min, add 1% green chain double antibody The normal saline was used to stop the digestion, and the cell pellet was collected; ②Continue to add collagenase, dispase and DNaseI to the pellet, shake and digest at 37°C for 2 hours, add green chain normal saline containing 1% double antibody to stop the digestion, and let it sta...
Embodiment 2
[0052] This embodiment provides a method for evaluating the hepatotoxicity of mitoxantrone based on a liver organoid model, comprising the following steps:
[0053] 1. Establishment of mouse liver organoid model:
[0054] 1) Sample cleaning: the mouse liver tissue was washed several times with normal saline containing 1% double antibody, and the obvious fat tissue was removed.
[0055] 2) Sample cutting: Cut the tissue into small pieces of about 0.5mm^3.
[0056] 3) Tissue digestion: ①Disperse the sheared tissue in normal saline, centrifuge to remove the supernatant, add collagenase, dispase and DNaseI to the precipitate, shake and digest at 37°C for 30min, add 1% green chain double antibody The normal saline was used to stop the digestion, and the cell pellet was collected; ②Continue to add collagenase, dispase and DNaseI to the pellet, shake and digest at 37°C for 2 hours, add green chain normal saline containing 1% double antibody to stop the digestion, and let it stand P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com