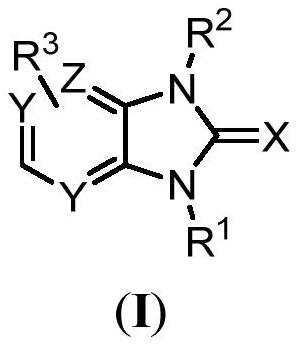
Benzimidazolone derivative or salt acceptable to agricultural pharmacology and application of benzimidazolone derivative or salt acceptable to agricultural pharmacology
A technology of benzimidazolone and its derivatives, which is applied in the field of benzimidazolone derivatives or pesticide acceptable salts, and can solve the problems of increasing the difficulty of pest control, serious drug resistance and exchange resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
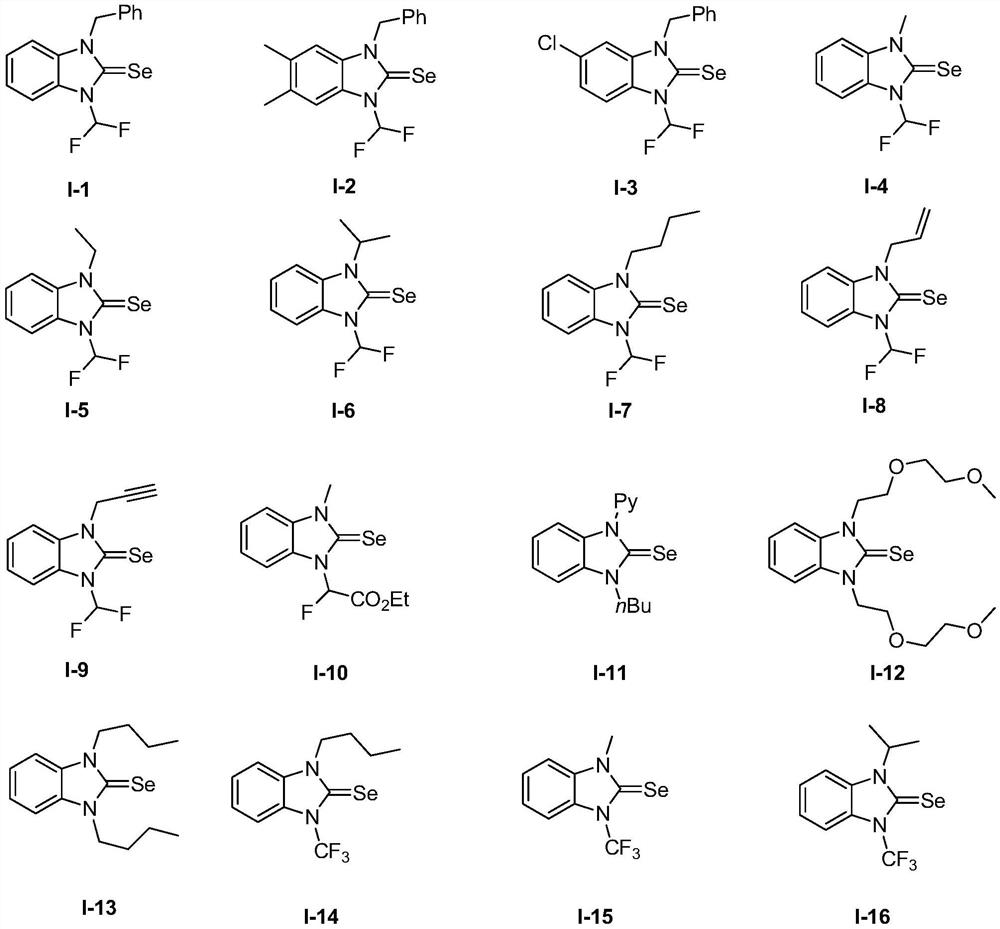
[0083] Embodiment 1: the preparation of compound I-10
[0084]
[0085] Add 1-methylbenzimidazole (0.40mmol) to the sealed tube, Na 2 S 2 o 4 (0.80mmol), selenium powder (0.80mmol), and dissolved with 2mL dichloroethane (DCE), then added ethyl bromofluoroacetate (1.00mmol), sealed the reaction tube and placed it in an oil bath at 80°C to stir the reaction 24h. After the reaction was monitored by TLC, the reaction mixture was cooled to room temperature, and the SO produced by the reaction was treated with NaOH aqueous solution. 2 , the reaction mixture was extracted with ethyl acetate, and the combined organic phases were washed with anhydrous Na 2 SO 4 After drying, the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (eluted with a mixed solvent of ethyl acetate and n-hexane) to obtain a white solid product with a yield of 62%.
[0086] Compounds I-22-I-56 were prepared by referring to the method of compound I-10.
Embodiment 2
[0087] Embodiment 2: the preparation of compound I-11
[0088]
[0089] Preparation method: under the protection of nitrogen, add 1-pyridyl-3-n-butyl-1H-benzo[d]imidazolium bromide (0.50mmol) to the solution containing Na 2 Se 2 (0.25mmol) in tetrahydrofuran solution (5mL), stirred at room temperature for 6-8h, then potassium tert-butoxide (0.50mmol) was added to the reaction solution, and stirred for 5-7h. After the reaction was monitored by TLC, the reaction mixture was cooled to room temperature, quenched by adding 3 mL of water, and extracted with ether, the organic phases were combined, and washed with anhydrous Na 2 SO 4 Dry and evaporate the solvent under reduced pressure on a rotary evaporator to obtain a yellow oily product, yield: 90%.
[0090] Compound I-12 was prepared by referring to the method of compound I-11.
Embodiment 3
[0091] Embodiment 3: the preparation of compound 1-13
[0092]
[0093] Put 1,3-dibutyl-1H-benzo[d]imidazolium bromide (0.50mmol) and selenium powder (2.50mmol) in a single-necked bottle, add 6mL of ether to dissolve, and put it at –78℃ for low temperature reaction Stir in the tank for 5min. NaHMDS (2.50 mmol) was dissolved in 4 mL of ether, and added dropwise to the reaction solution under ice-bath stirring. After stirring at -78°C for 30 min, the stirring reaction was continued at room temperature for 16 h. After the reaction was monitored by TLC, the reaction solution was cooled to room temperature, and the solvent was evaporated under reduced pressure. The residue was purified by column chromatography (eluted with a mixed solvent of dichloromethane and n-hexane) to obtain a yellow solid with a yield of 76%.
[0094] Compound I-14 was prepared according to the method of compound I-13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com