Porcine fibrinogen freeze-dried preparation, preparation process and application of porcine fibrinogen freeze-dried preparation
A technology for fibrinogen and freeze-dried preparations, which is applied in the field of pig-derived fibrin freeze-dried preparations, can solve the problems that the reconstitution time cannot be effectively improved, and achieve the effects of shortening the preparation time, short reconstitution time, and good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
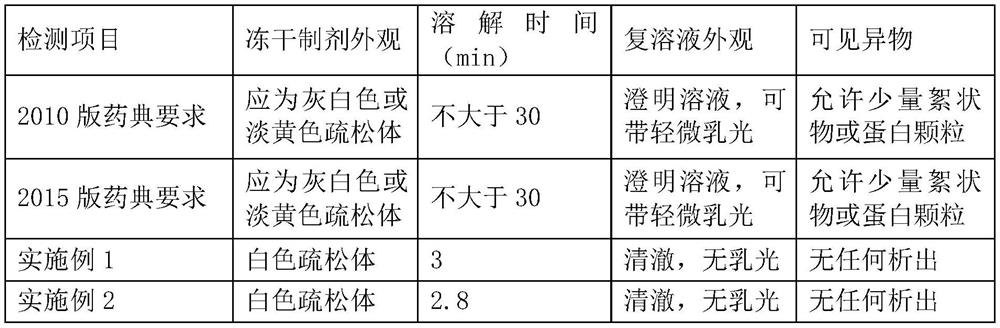
Embodiment 1
[0025] 1. A freeze-dried preparation of porcine-derived fibrinogen, calculated per mL of preparation, comprising:
[0026] Pig-derived fibrinogen 100mg, sodium chloride 12mg, sodium citrate 10mg, arginine hydrochloride 90mg, mannitol 1mg, poloxamer 188 1mg, trehalose 0.5mg, sodium alginate 0.5mg.
[0027] 2, the preparation technology of described porcine source fibrinogen freeze-dried preparation, comprises as follows:
[0028] The precipitate obtained by cold ethanol precipitation of pig plasma is pig-derived fibrinogen. After the precipitate is rinsed with water for injection, it is dissolved with a buffer solution containing sodium citrate, sodium chloride, and arginine hydrochloride (buffer solution formula: citric acid Sodium 10g / L, sodium chloride 12g / L, arginine hydrochloride 90g / L; use hydrochloric acid or sodium hydroxide to adjust the pH to 7.0), stir evenly for 1-2 hours, then add mannitol, poloxamer 188, Trehalose, sodium alginate, so that the concentrations of m...
Embodiment 2
[0036] 1. A freeze-dried preparation of porcine-derived fibrinogen, calculated per mL of preparation, comprising:
[0037] Pig-derived fibrinogen 100mg, sodium chloride 6mg, sodium citrate 5mg, arginine hydrochloride 60mg, polysorbate 201.2mg, sucrose 0.5mg, sodium alginate 0.5mg.
[0038] 2, the preparation technology of described porcine source fibrinogen freeze-dried preparation, comprises as follows:
[0039] The precipitate obtained by cold ethanol precipitation of pig plasma is pig-derived fibrinogen. After the precipitate is rinsed with water for injection, it is dissolved with a buffer solution containing sodium citrate, sodium chloride, and arginine hydrochloride (buffer solution formula: citric acid Sodium 5g / L, sodium chloride 6g / L, arginine hydrochloride 60g / L; use hydrochloric acid or sodium hydroxide to adjust the pH to 7.0), stir evenly for 1-2 hours, then add polysorbate 20, sucrose, alginic acid Sodium, so that the concentrations of polysorbate 20, sucrose, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com