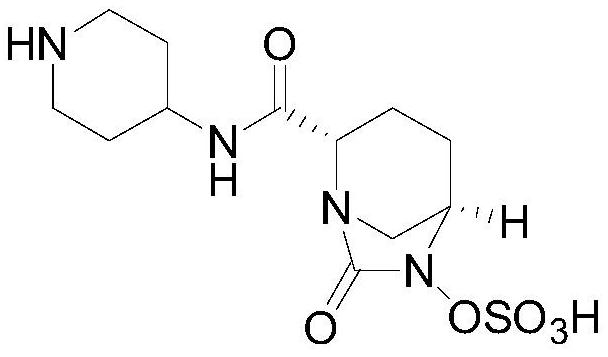
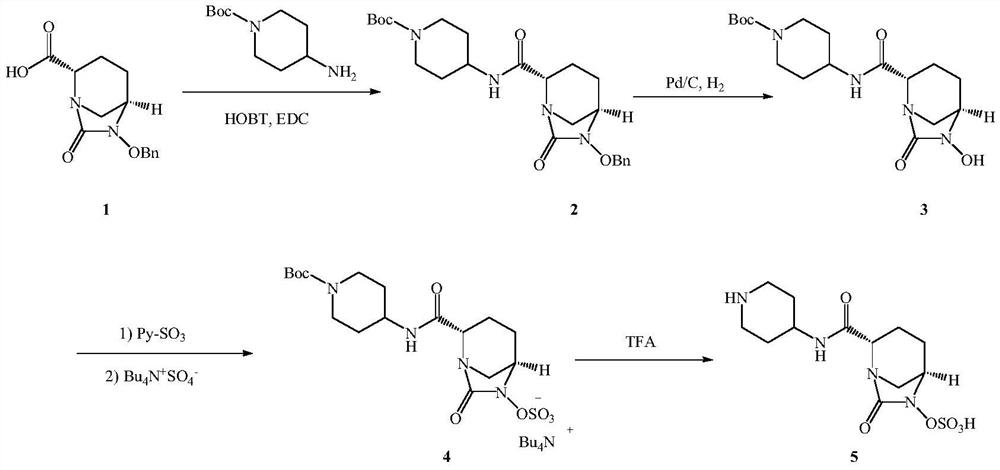
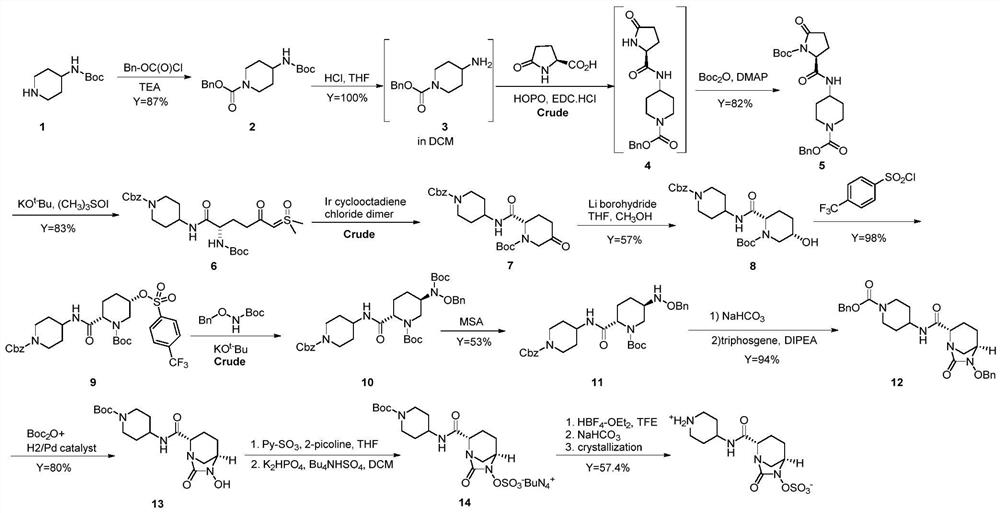
A kind of preparation method of relebactam
A technology for reilobactam and intermediates, which is applied in the production of organic chemistry and bulk chemicals, can solve the problem of not getting rid of high-pressure catalytic hydrogenation, etc., and achieve the effects of safety and environmental protection in the synthesis process, reduction of process operations, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] (1) Intermediate 3: ethyl (2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3,2,1]octane-2-carboxylate
[0059] Add 100ml of ethyl acetate and 10.0g of compound 2 into the reaction flask, and start stirring. Slowly add 5% sodium carbonate solution at room temperature until the solid basically disappears, stir for 30-40 minutes, let stand to separate layers; keep the organic layer, add anhydrous sodium sulfate to dry for 1-2 hours. Filter and concentrate to half volume. Add 8.2 g of triethylamine; control the temperature at 5±5° C., and add dropwise 8.1 g of triphosgene in ethyl acetate (20 ml of ethyl acetate). After the dropwise addition, control the temperature at 5±5°C, stir and react for 1 hour, add 100ml of purified water, control the reaction temperature at 25±5°C, stir and react for 0.5 to 1 hour, and then stand to separate layers. The organic layer was retained and concentrated under reduced pressure; 8.1 g of a reddish-brown solid was obtained, namely intermediate...
Embodiment 2
[0069] (1) Intermediate 3: ethyl (2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3,2,1]octane-2-carboxylate
[0070] Add 100ml of dichloromethane and 10.0g of compound 2 into the reaction flask, and start stirring. Slowly add 5% sodium carbonate solution at room temperature until the solid basically disappears, stir for 30-40 minutes, let stand to separate layers; keep the organic layer, add anhydrous sodium sulfate to dry for 1-2 hours. Filter and concentrate to half volume. Add 8.2 g of triethylamine; control the temperature at 5±5° C., and add 7.2 g of triphosgene in dichloromethane solution (20 ml of dichloromethane) dropwise. After the dropwise addition, control the temperature at 5±5°C, stir and react for 1 hour, add 100ml of purified water, control the reaction temperature at 25±5°C, stir and react for 0.5 to 1 hour, and then stand to separate layers. The organic layer was retained and concentrated under reduced pressure; 8.0 g of a reddish-brown solid was obtained, name...
Embodiment 3
[0080] (1) Intermediate 3: ethyl (2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3,2,1]octane-2-carboxylate
[0081] Add 1000ml of dichloromethane and 100.0g of compound 2 into the reaction flask, and start stirring. Slowly add 5% sodium carbonate solution at room temperature until the solid basically disappears, stir for 30-40 minutes, let stand to separate layers; keep the organic layer, add anhydrous sodium sulfate to dry for 1-2 hours. Filter and concentrate to half volume. Add 82.3g of triethylamine; control the temperature at 5±5°C, and add 72.1g of triphosgene in dichloromethane solution (200ml of dichloromethane) dropwise. After the dropwise addition, control the temperature at 5±5°C, stir and react for 1 hour, add 1000ml of purified water, control the reaction temperature at 25±5°C, stir and react for 0.5 to 1 hour, and then stand to separate layers. The organic layer was retained and concentrated under reduced pressure; 80.5 g of a reddish-brown solid was obtained, na...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com