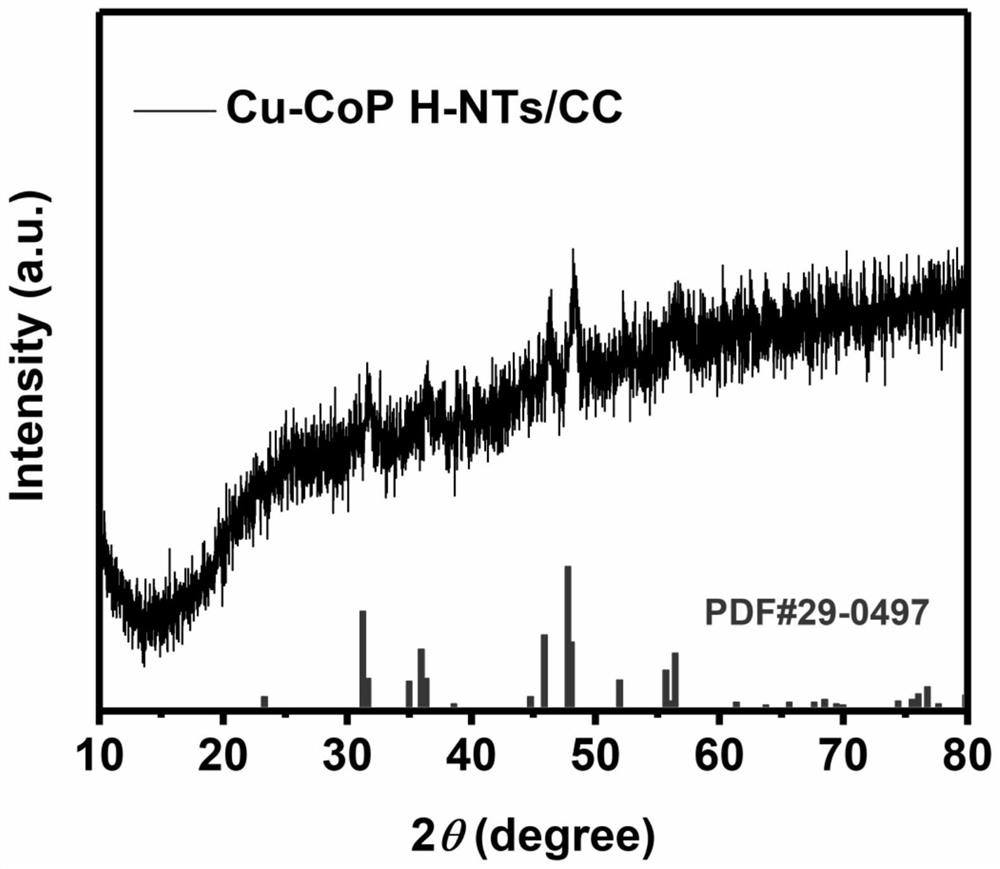
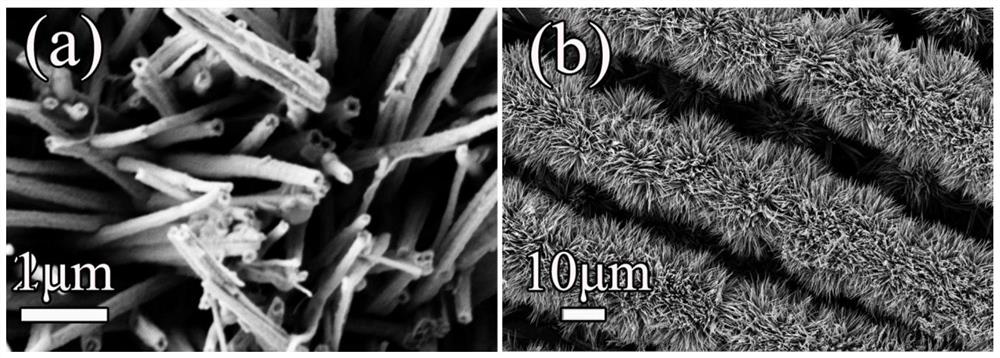
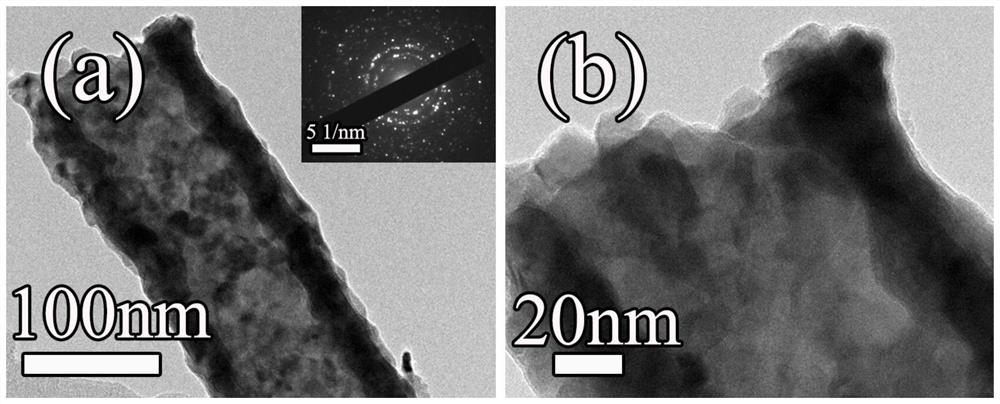
Copper-doped cobalt phosphide bifunctional water electrolysis catalytic material in hollow nanotube structure
A catalytic material, copper-doped technology, applied in physical/chemical process catalysts, electrolysis processes, electrolysis components, etc., can solve the problems of OER catalyst scarcity and cost, achieve large electrochemical surface area and electrochemical active sites, stability The effect of strong, huge industrial and commercial value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Step 1. Preparation of copper-doped cobalt oxide and cobalt hydroxide precursors grown on carbon cloth by hydrothermal process:
[0039] Weigh 0.2 mM C 4 h 6 CuO 4 ·H 2 O (copper acetate monohydrate), 2 mM Co(NO 3 ) 2 ·6H 2 O (cobalt nitrate hexahydrate), 6 mM H 4 FN (ammonium fluoride), 10 mM CH 4 N 2 O (urea), it was added to 30mL deionized water, and magnetically stirred for 20 min to ensure that the salt solution was evenly mixed. Add the precursor mixed solution and a piece of pretreated 1cm×4cm carbon cloth into a stainless steel autoclave with polytetrafluoroethylene, and then transfer it to an electric blast drying oven, set the hydrothermal temperature to 120°C , water heating time 6h. After the hydrothermal treatment, the surface of the carbon cloth was cleaned with deionized water and then dried at 60°C for 6 hours.
[0040] Step two, phosphating:
[0041] The phosphating process is carried out in a CVD tube furnace. 5mM sodium hypophosphite is pl...
Embodiment 2
[0043] Step 1. Preparation of copper-doped cobalt oxide and cobalt hydroxide precursors grown on carbon cloth by hydrothermal process:
[0044] Weigh 0.3 mM C 4 h 6 CuO 4 ·H 2 O (copper acetate monohydrate), 3 mM Co(NO 3 ) 2 ·6H 2 O (cobalt nitrate hexahydrate), 9 mM H 4 FN (ammonium fluoride), 15 mM CH 4 N 2 O (urea), it was added to 30mL deionized water, and magnetically stirred for 20 min to ensure that the salt solution was evenly mixed. Add the precursor mixed solution and a piece of pretreated 1cm×4cm carbon cloth into a stainless steel autoclave with polytetrafluoroethylene, and then transfer it to an electric blast drying oven, set the hydrothermal temperature to 120°C , water heating time 6h. After the hydrothermal treatment, the surface of the carbon cloth was cleaned with deionized water and then dried at 60°C for 6 hours.
[0045] Step two, phosphating:
[0046] The phosphating process is carried out in a CVD tube furnace. 5mM sodium hypophosphite is pl...
Embodiment 3
[0048] Step 1. Preparation of copper-doped cobalt oxide and cobalt hydroxide precursor grown on carbon cloth by hydrothermal process:
[0049] Weigh 0.2 mM C 4 h 6 CuO 4 ·H 2 O (copper acetate monohydrate), 2 mM Co(NO 3 ) 2 ·6H 2 O (cobalt nitrate hexahydrate), 6 mM H 4 FN (ammonium fluoride), 10 mM CH 4 N 2 O (urea), it was added to 30mL deionized water, and magnetically stirred for 20 min to ensure that the salt solution was evenly mixed. Add the precursor mixed solution and a piece of pretreated 1cm×4cm carbon cloth into a stainless steel autoclave with polytetrafluoroethylene, and then transfer it to an electric blast drying oven, set the hydrothermal temperature to 120°C , water heating time 6h. After the hydrothermal treatment, the surface of the carbon cloth was cleaned with deionized water and then dried at 60°C for 6 hours.
[0050] Step 2, phosphating process to prepare the final copper-doped cobalt phosphide bifunctional water electrolysis catalytic mater...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com