Process for preparing cross-linked phthalocyanine polymer and its uniformly transparent film
A phthalocyanine and thin film technology, applied in the field of preparing the above-mentioned prepolymer thin film, can solve the problems of high temperature resistance, organic solvent resistance and acid and alkali resistance that are inferior to phthalocyanine, inability to adapt, and film performance decline.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
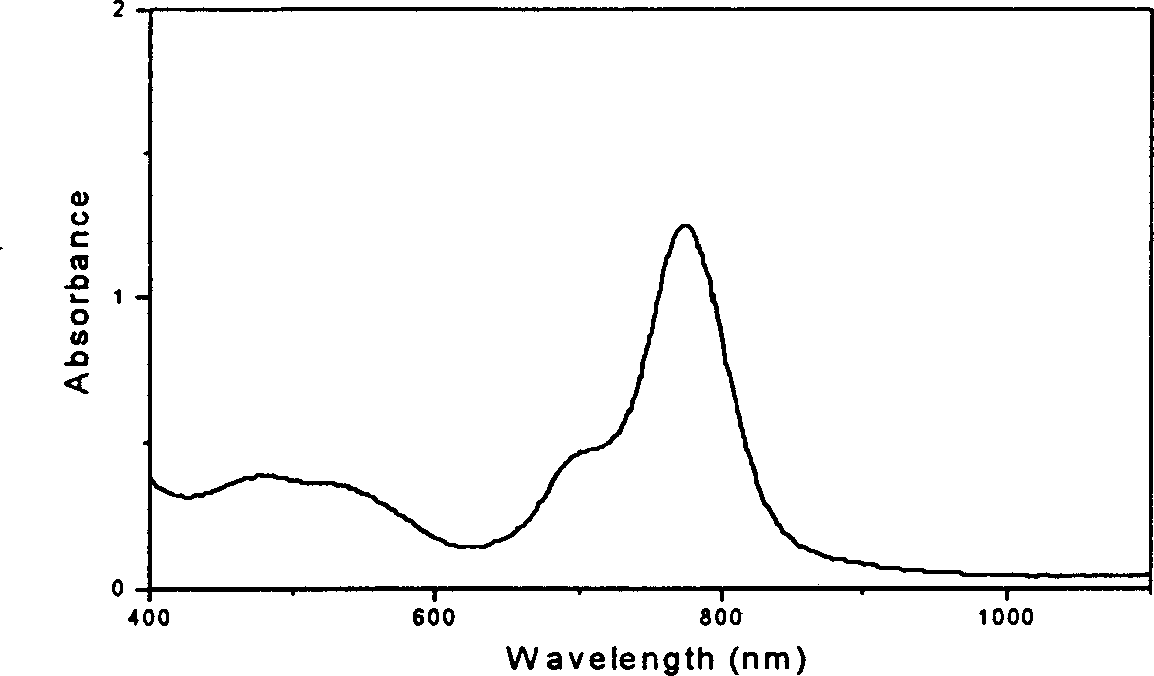
[0019] Figure 6 is the thermal stability curve of polymer 2 in Example 2. Among them, the abscissa is the temperature, and the ordinate is the maximum absorption value. The heating time for each temperature was 5 minutes. Embodiment 1,2,9,16,23-tetraaminovanadyl phthalocyanine-epoxy resin crosslinked polymer (polymer 1) and its film
[0020] Synthesis of 4-nitrophthalimide: 16.8ml of fuming nitric acid and 196ml of concentrated sulfuric acid were mixed and cooled to 12°C. Then 14.0 g of phthalimide was added thereto as quickly as possible with stirring, keeping the temperature of the mixture between 10°C and 15°C overnight. The resulting clear solution was poured into 320 g of crushed ice with vigorous stirring so that the temperature of the mixture did not exceed 20°C. Suction filter with a glass sand funnel, wash the solid matter with cold water several times, and then dry. A crude product of 12.8 g was obtained. The crude product was recrystallized with 95% ethanol t...
Embodiment 2、1
[0032] Thermal Stability Analysis: Figure 4 is the variation of the maximum absorption value of polymer 1 film sample with temperature. Wherein, the residence time of each temperature is 5 minutes. It can be seen that the absorption peak of the sample begins to decay at about 200°C, and decays by 53% at 260°C. Embodiment 2,1,8,15,22-tetraaminovanadyl phthalocyanine-epoxy resin crosslinked polymer (polymer 2) and its film
[0033] Synthesis of 3-nitrophthalic anhydride: Mix 13ml concentrated sulfuric acid and 10g phthalic anhydride, heat at 80°C, after the solution is clarified, slowly drop 6ml fuming nitric acid (specific gravity 1.5 l), keeping the temperature of the reactants between 100 and 110°C. After adding nitric acid, keep this temperature for 2h. The reactant was poured into 20ml of water and cooled. Add 4ml of water to the obtained solid substance, stir well, and filter with suction. The crude product was recrystallized twice with 4ml of water, and air-dried t...
Embodiment 3、2
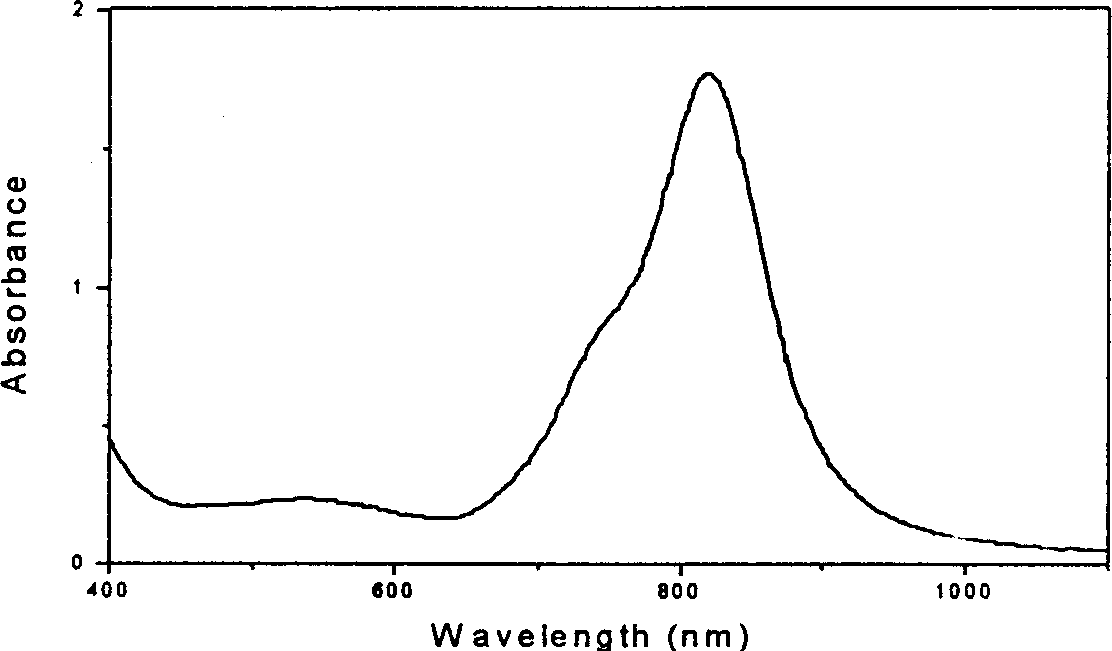
[0042] Thermal Stability Analysis: Figure 6 is the variation of the maximum absorption value of the polymer 2 film sample with temperature. It can be seen that the absorption peak of the sample begins to decay at about 200°C, and decays by 55% at 260°C. Embodiment 3,2,9,16,23-tetraaminocopper phthalocyanine-epoxy resin crosslinked polymer (polymer 3) and its film
[0043] Synthesis of 2,9,16,23-tetraaminocopper phthalocyanine: 1.2g (6.25×10 -3 mol) 4-nitrophthalimide, 2.0g (0.033mol) urea, 0.15g (1.5×10 -3 mol) cuprous chloride, 0.2g (0.0037mol) ammonium chloride and 0.02g (1.6×10 -5 mol) ammonium molybdate was reacted in 2ml of nitrobenzene at 180°C for 6h, suction filtered, and the solid matter was washed with hot methanol, hot sodium carbonate aqueous solution, hot 1M hydrochloric acid solution and hot water respectively, and the resulting product was directly dissolved in 20ml of 5% sodium sulfide The aqueous solution was refluxed for 20 h, and the product was washed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com