Heterobicyclic inhibitors of mat2a and methods of use for treating cancer
A heterocycloalkyl and carbocyclyl technology, applied in the field of heterobicyclic inhibitors of MAT2A and in the treatment of cancer, can solve problems such as gene inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
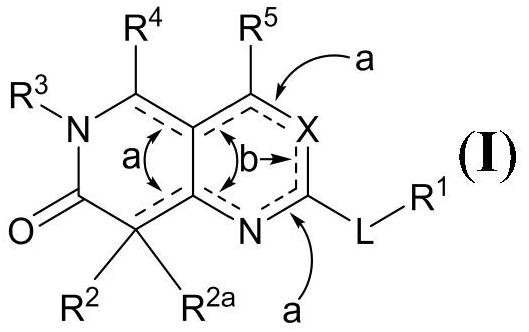
[0085] According to various embodiments, the compound of formula (I) has a structure according to formula (IA):
[0086]
[0087] Alternatively, the compound of formula (I) has a structure according to formula (IB):
[0088]
[0089] The present disclosure also provides compounds according to formula II and pharmaceutically acceptable salts thereof:
[0090]
[0091] In Formula II, L is O, S, NR, or a bond; and R is H or C 1 -C 6 -alkyl.
[0092] R 1 Selected from: C 1 -C 6 -Alkyl, C 2 -C 6 -Alkenyl, C 3 -C 6 -carbocyclyl, -(C 1 -C 6 -Alkyl)(C 3 -C 6 -carbocyclyl) and -(C 1 -C 6 -Alkyl)(C 3 -C 6 -cycloalkenyl), wherein R 1 Any alkyl in is linear or branched, and R 1 Optionally substituted with 1-6 halo.
[0093] Alternatively, in some embodiments, when L is NR, then R and R 1 Combination with L represents optionally one or more R A Substituted 3- to 6-membered heterocycloalkyl (wherein 1-4 ring members are independently selected from: N, O and S)...
Embodiment
[0186] The present invention will be more fully understood by reference to the following examples. However, the examples provided herein are illustrative and should not be construed as limiting the scope of the present disclosure.
[0187] List of abbreviations and terms:
[0188] anhy. anhydrous
[0189] aq. Water-based
[0190] min minutes
[0191] mL milliliter
[0192] mmol millimole
[0193] mole mole
[0194] MS mass spectrometry
[0195] NMR nuclear magnetic resonance
[0196] TLC thin layer chromatography
[0197] HPLC high performance liquid chromatography
[0198] RT(r.t.) room temperature
[0199] -spectrum
[0200] Hz hertz
[0201] δ chemical shift
[0202] J coupling constant
[0203] s unimodal
[0204] d doublet
[0205] t triplet
[0206] q quartet
[0207] m multiplet
[0208] br broad peak
[0209] qd quadruple doublet
[0210] dquin double quintet
[0211] dd double doublet
[0212] dt double triplet
[0213] Solvents and reagents: ...
preparation Embodiment 101
[0259]
[0260]Step A: 1-tert-butyl 3-ethyl 3-(5-(dimethoxymethyl)-2-(methylthio)pyrimidin-4-yl)pyrrolidine-1,3-dicarboxylate
[0261]
[0262] To a solution of 1-tert-butyl 3-ethyl pyrrolidine-1,3-dicarboxylate (4.8 g, 19.8 mmol, 1.5 equiv) in THF (30 mL) over a period of 1 h at -78 °C via an addition funnel LiHMDS (1M in THF, 26.4 mL, 26.4 mmol, 2.0 equiv) was added to LiHMDS. The mixture was stirred at -78°C for 4 hours. Then a solution of 4-chloro-5-(dimethoxymethyl)-2-(methylthio)pyrimidine (3.1 g, 13.2 mmol, 1.0 equiv) in THF (10 mL) was added. The reaction mixture was warmed to room temperature and stirred for an additional 3 hours. Then the reaction mixture was washed with NH 4 Quenched with Cl(sat. aq.) (50 mL) and extracted with EtOAc (50 mL x 3). The organic layers were combined and washed with Na 2 SO 4 Dry, filter, concentrate and the resulting residue is purified by flash column chromatography on silica gel (PE / EtOAc=20 / 1 to 4 / 1) to give 3-(5-(dimetho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com