Biodegradable cryogel dressing as well as preparation method and application thereof
A biodegradable, gelatinous technology, applied in the field of biomedical materials, can solve the problems of hemostatic materials that cannot be used, massive bleeding, and incompressibility, and achieve the effects of avoiding secondary bleeding and pain, promoting vascularization, and being easy to store
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
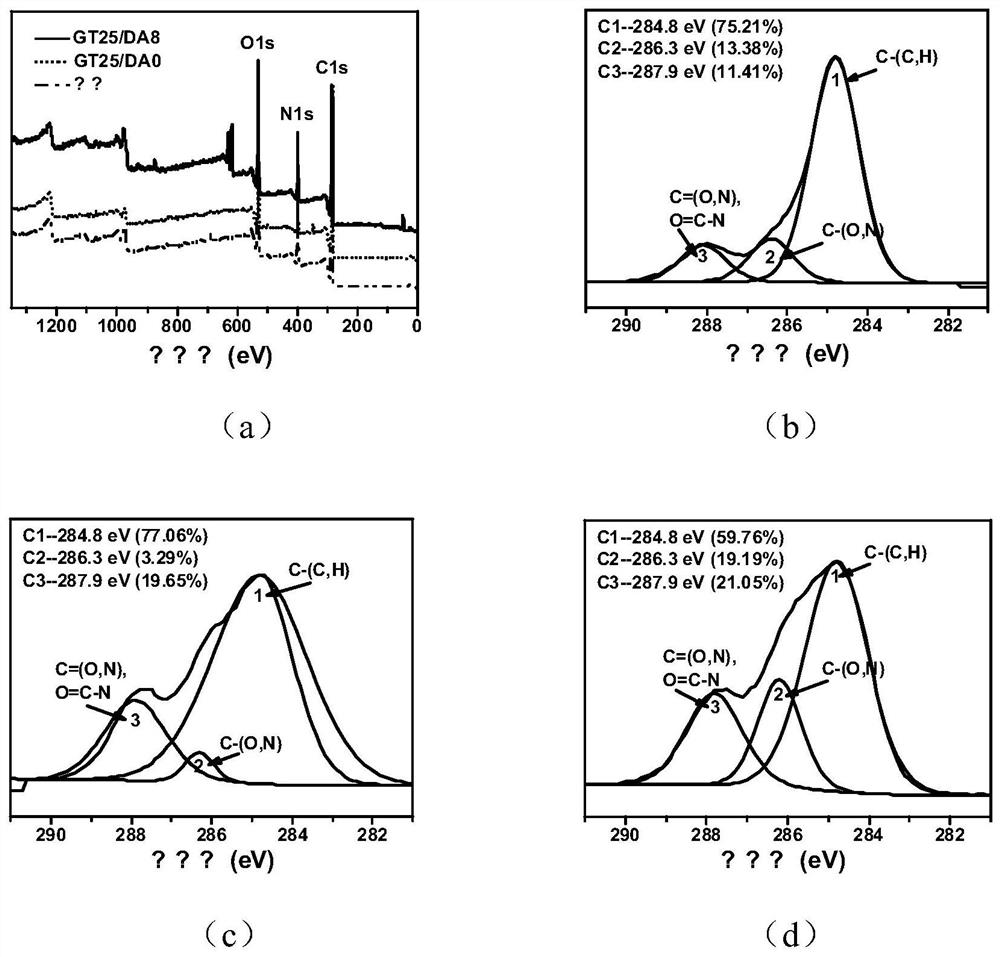
[0052] Preparation of GT25 / DA0 crystal gel dressing: adding gelatin (GT) to deionized water to configure a 5.0wt% GT solution; adding 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (EDC hydrochloride) was added to deionized water to configure a 75mg / mL EDC solution; N-hydroxysuccinimide (NHS) was added to deionized water to configure a 45mg / mL NHS solution; subsequently, Place the GT solution, EDC solution, NHS solution and deionized water in an ice-water mixing bath to fully pre-cool and then fully mix to obtain a final concentration of GT of 2.5wt%, wherein the amino groups on the gelatin, EDC and NHS and the molar The ratio was 1:0.5:0.5, and then the mixture was placed in a low-temperature reactor at -12°C for 36 hours. After the reaction, the crystal gel dressing was thawed, dialyzed in deionized water for 1 day, and freeze-dried at -80°C to obtain the biodegradable crystal gel dressing GT25 / DA0.
Embodiment 2
[0054] Preparation of GT25 / DA2 crystal gel dressing: adding gelatin to deionized water to configure 5.0wt% GT solution; 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride Add to deionized water to make 75mg / mL EDC solution; add N-hydroxysuccinimide to deionized water to make 45mg / mL NHS solution; add dopamine hydrochloride to deionized water to make 100mg / mL DA solution; sodium periodate was added to deionized water to form a 37.6 mg / mL SP solution; subsequently, GT solution, EDC solution, NHS solution, DA solution, SP solution and deionized water were placed in an ice-water mixing bath Fully pre-cooled and then fully mixed to obtain a final concentration of GT of 2.5wt%, a final concentration of DA of 2.0mg / mL, wherein, the molar ratio of the amino group on the gelatin, EDC and NHS is 1:0.5:0.5, periodate The molar ratio of sodium to dopamine is 1:1. Then the mixture was placed in a low-temperature reactor at -12°C for 36 hours of reaction. After the reaction, the ...
Embodiment 3
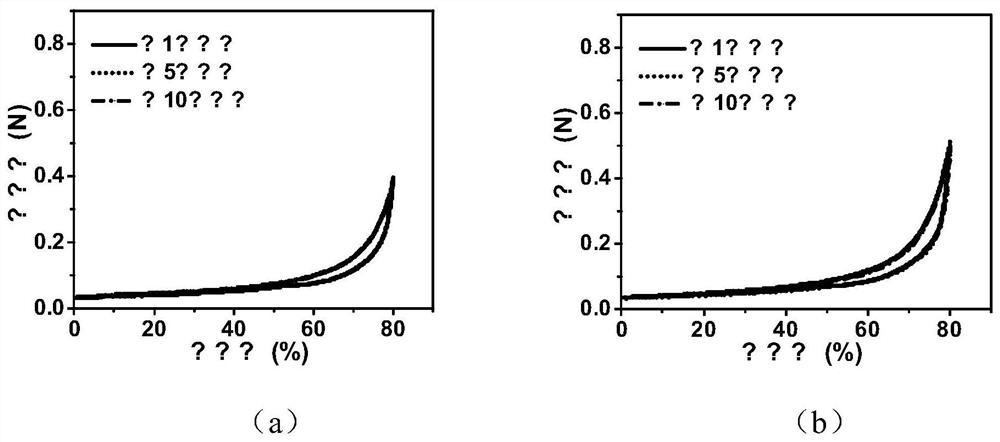
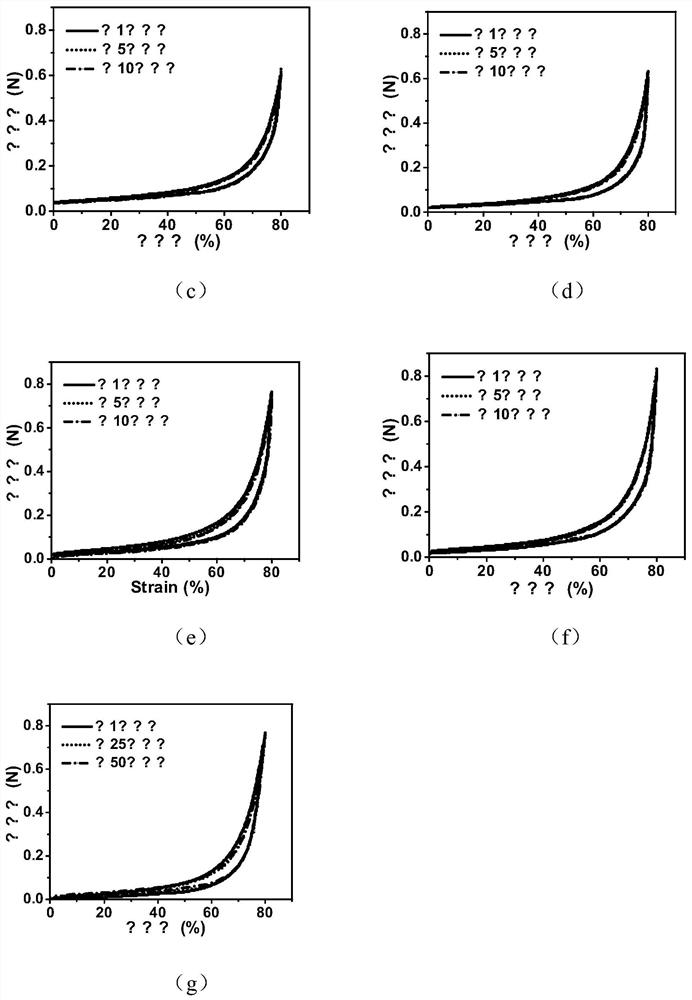
[0056] The final concentration of DA in Example 2 is controlled at 4.0mg / mL, 6.0mg / mL, 8.0mg / mL and 10.0mg / mL respectively, and other conditions are the same as in Example 2, and the biodegradable gel dressing GT25 can be obtained respectively / DA4, GT25 / DA6, GT25 / DA8 and GT25 / DA10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com