Hindered phenol antioxidant with new structure and preparation method thereof
A hindered phenol antioxidant and a new structure technology are applied in the field of the new structure hindered phenol antioxidant and its preparation, which can solve the problems of difficulty in synthesis and reduce the application amount, and achieve the effects of increasing the dispersion effect, and the synthesis process is simple and environmentally friendly.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
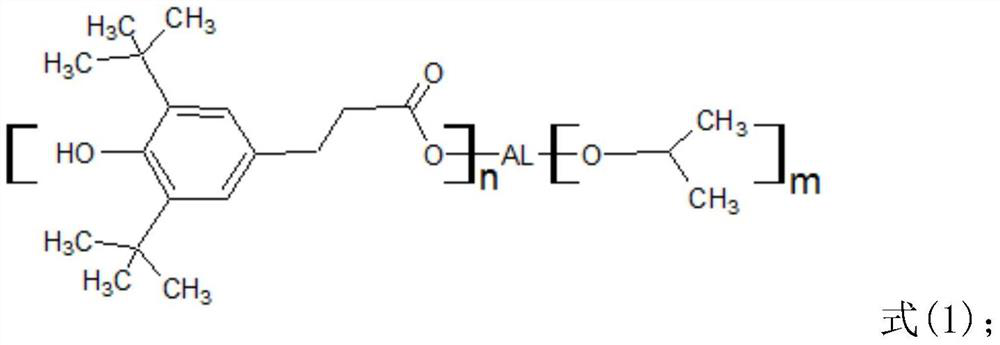
[0034] The invention provides a hindered phenol antioxidant I with a new structure, which has a structure shown in formula (1):
[0035]
[0036] Wherein, n is an integer of 1-3, and m is an integer of 0-2.
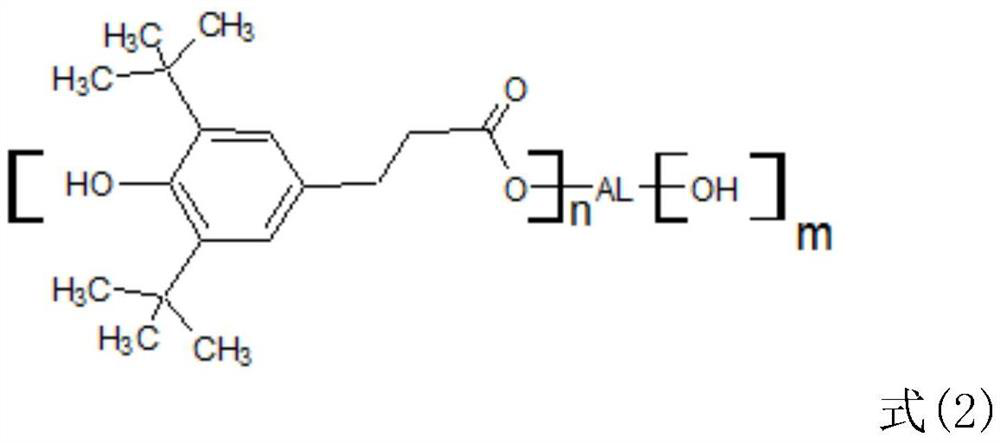
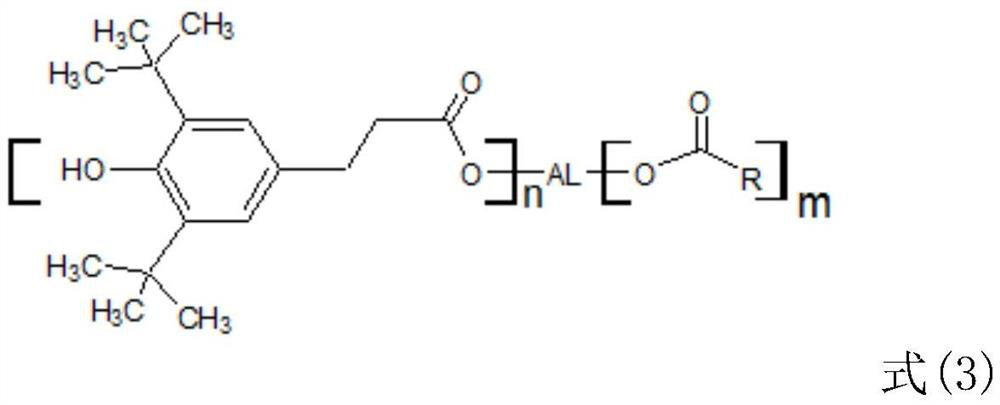
[0037] Specifically, its structure is the following three:
[0038]
[0039] Wherein, n is 1, and m is 2.
[0040]
[0041] Wherein, n is 2, and m is 1.
[0042]
[0043] Among them, n is 3, and m is 0.
[0044] Another aspect of the present invention provides a kind of method of new structure hindered phenolic antioxidant I, comprises the following steps:
[0045] 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid and a certain amount of aluminum isopropoxide are reacted in an organic solvent at a certain temperature for a certain period of time to form a new structure hindered phenol antioxidant I; Described organic solvent is benzene, toluene, xylene, cyclohexane, pentane, hexane, heptane, octane, cyclohexanone, chlorobenzene, dichlorobenzene, methylen...
Embodiment 2
[0076] In order to set forth the present invention more clearly, concrete experiment will be adopted to analyze below:
[0077] ①Preparation of hindered phenol antioxidant I with new structure:
[0078] Add 27.7 grams of 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid, 6.8 grams of aluminum isopropoxide and 100 milliliters of toluene into the reaction flask, stir and heat to 85-90 ° C, keep micro-distillation Remove the reaction by-product isopropanol, react for about 2 hours, add 100 ml of water to distill and recover toluene after the reaction is completed, filter and dry to obtain tri[3-(3,5-di-tert-butyl-4-hydroxyphenyl)propane acid] aluminum (compound 3:C 51 h 75 o 9 AL).
[0079] ②Preparation of hindered phenol antioxidant II with new structure:
[0080] Add 27.7 grams of 3-(3,5-di-tert-butyl-4-hydroxyphenyl) propionic acid, 10.2 grams of aluminum isopropoxide and 100 milliliters of toluene into the reaction flask, stir and heat to 85-90 ° C, keep micro-distill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com