A kind of polypeptide with antitumor activity and its application
A technology of anti-tumor activity and anti-tumor drugs, applied in the field of polypeptides with anti-tumor activity, to achieve the effect of short polypeptide sequence, broad clinical application value and prospects, and significant anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: pET-30a / (His) 6 - Construction of LHRHR protein expression vector
[0020] Such as figure 1 Construct pET-30a / (His) as indicated 6 -LHRHR, search the gene sequence of LHRHR (1-125AA, NM_000233.4) from the Genbank database, design PCR primers, the upstream primer sequence is: 5'-CG GGATCC ATGAAGCAGCGGTTCTCGGC-3' (BamHI), the downstream primer sequence is: 5'-CC AAGCTT GCTCCGGGCTCAATGTATCT-3' (HindⅢ), the underlined part is the restriction site sequence. The PCR product and the vector pET-30a were digested with BamHI and Hind III at 37°C for 3 h, then ligated with T4 DNA ligase at 16°C for 12 h. Transform the ligation product into DH5α competent cells, then spread the transformation product on a kanamycin-resistant (50 μg / ml) LB plate and culture until a single colony grows, pick a single colony, and extract the plasmid for enzyme digestion verification , the recombinant plasmid was sequenced to obtain the recombinant plasmid pET-30a / (His) 6 - LHRHR.
Embodiment 2
[0021] Example 2: (His) 6 - Expression, purification and validation of LHRHR
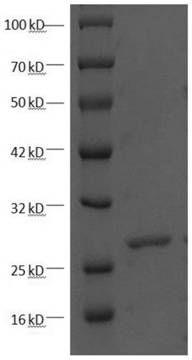
[0022] The recombinant plasmid pET-30a / (His) prepared in Example 1 6 -LHRHR was transformed into BL21 (DE3) strain, and the recombinant plasmid was screened on a kanamycin-resistant LB plate, and cultured to OD in 10 ml LB liquid medium (containing 50 μM kanamycin) 600 After about 0.5, the culture was inoculated in multiple bottles of LB liquid medium at a volume ratio of 1: 10, and cultured to OD at 37°C with vigorous shaking 600 About 0.5, add isopropyl-β-D-thiogalactopyranoside (IPTG) at a final concentration of 1 mM and induce at 37 °C for 10 h to obtain a bacterial solution.
[0023] Centrifuge the bacterial liquid, remove the supernatant, and resuspend the bacterial pellet in the lysate (50 mM Tris–HCl, 20 mM imidazole, 100 mM NaCl, 10% glycerol , 1% Triton, 1 mM protease inhibitor PMSF, 1 mg / ml lysozyme, pH 8.0), placed on ice for 30 min, sonicated, and centrifuged at 12000 g for 30 min to...
Embodiment 3
[0025] Example 3: Phage display panning for bioactive peptides specifically binding to LHRHR
[0026] (1) Immobilize the target protein: mix 600 μl of the target protein solution with a concentration of 17 μg / ml (0.1 M NaHCO 3 pH8.6) was added to a six-well plate, placed on a shaker with slight shaking, and incubated overnight at 4°C. After washing 6 times with TBST (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 0.1% [v / v] Tween-20 ), blocked with blocking solution (0.1 M NaHCO 3 pH 8.6, 5 mg / ml BSA, 0.02% NaN 3 ) closed for 1 h.
[0027] (2) Screening for specifically binding phages: Wash the six-well plate 10 times with TBST. The amplified phage was diluted with TBST to a copy number of 10 9 ~10 11 In between, the diluted phage was added to the six-well plate to allow it to bind to the target protein, and incubated at room temperature for about 60 min. Wash 10 times with TBST and pat dry after each wash. Add eluent to collect phages that specifically bind to the target protei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com