Near-infrared light stimulation response type functional macromolecule, intelligent nanometer material and preparation method of intelligent nanometer material
A near-infrared light, stimuli-responsive technology, applied in steroids, organic chemistry, etc., can solve the problem of less intelligent nanomaterials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis of the target molecule Chol-CroA-Chol:
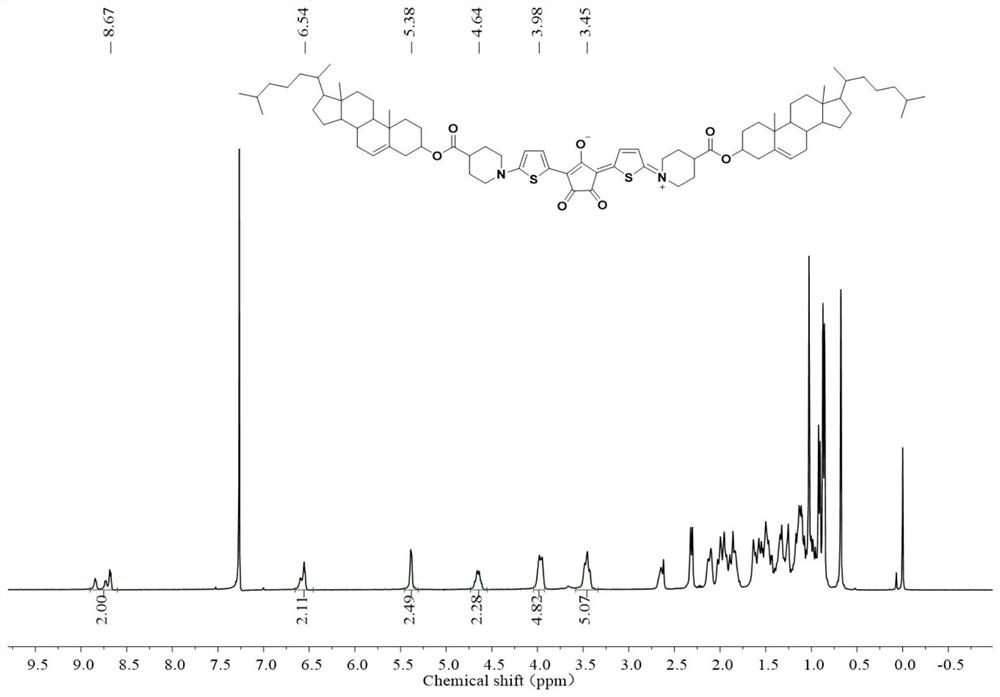
[0068] Add carboxylic acid-piperidine-thiophene and cholesterol to a dry 50mL reaction flask, add dry dichloromethane and stir to dissolve, then add the catalyst DMAP, replace nitrogen three times and keep the nitrogen atmosphere, add EDC after half an hour, under the protection of inert gas The reaction was stirred at room temperature for 24 h. After the reaction is completed, it is washed, dried and filtered, the solvent is removed by rotary evaporation, and purified by column chromatography to obtain cholesterol-piperidine-thiophene. Add croconic acid and cholesterol-piperidine-thiophene to a dry 25mL reaction flask, add a dry mixed solvent of toluene and n-butanol, and heat to reflux for 2h. After the reaction was completed, the solvent was removed by rotary evaporation and purified by column chromatography to obtain the target molecule Chol-CroA-Chol. See the H NMR spectrum figure 1 .
Embodiment 2
[0070] Synthesis of the target molecule Boc-NH-CroA-NH-Boc.
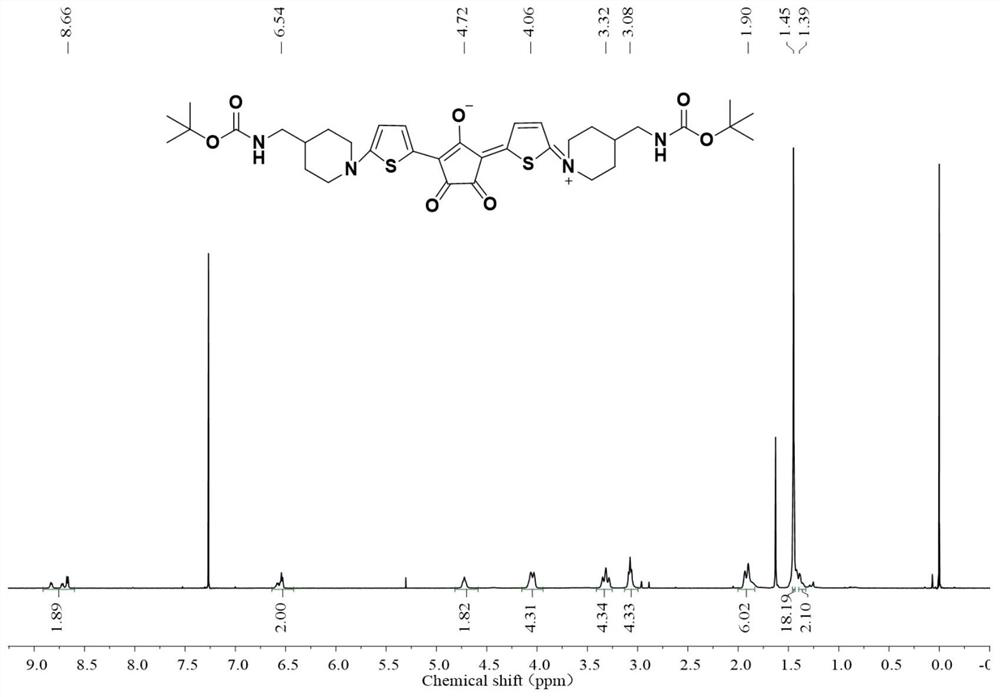
[0071] Add Boc-aminomethyl-piperidine and 2-mercapto-thiophene to a dry 50mL reaction flask, add dry toluene and stir to dissolve, replace the nitrogen three times and keep the nitrogen atmosphere, and heat to reflux for 6h. After the reaction, the solvent was removed by rotary evaporation, and purified by column chromatography to obtain Boc-aminomethyl-piperidine-thiophene. Add Boc-aminomethyl-piperidine-thiophene and croconic acid to a dry 25mL reaction flask, add a dry mixed solvent of toluene and n-butanol, and heat to reflux for 2h. After the reaction was completed, the solvent was removed by rotary evaporation and purified by column chromatography to obtain the target molecule Boc-NH-CroA-NH-Boc. See the H NMR spectrum figure 2 .
Embodiment 3
[0073] Synthesis of target molecule Me-DEG-CroA-DEG-Me
[0074] Add carboxylic acid-piperidine-thiophene and diethylene glycol monomethyl ether to a dry 50mL reaction bottle, add dry dichloromethane and stir to dissolve, then add catalyst DMAP, replace nitrogen three times and keep nitrogen atmosphere, add after half an hour EDC, under the protection of an inert gas, stirred at room temperature for 24 h. After the reaction is completed, wash, dry and filter, remove the solvent by rotary evaporation, and purify by column chromatography to obtain diethylene glycol monomethyl ether-piperidine-thiophene. Add croconic acid and diethylene glycol monomethyl ether-piperidine-thiophene to a dry 25mL reaction flask, add a dry mixed solvent of toluene and n-butanol, and heat to reflux for 2h. After the reaction was completed, the solvent was removed by rotary evaporation and purified by column chromatography to obtain the target molecule Me-DEG-CroA-DEG-Me. See the H NMR spectrum ima...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com