Anti-pd-1/lag3 bispecific antibodies
A bispecific antibody, PD-1 technology, applied in anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, anti-animal/human immunoglobulin, specific peptide, etc., can solve tumor immune response Impaired, weakened T cell activation immune surveillance and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0375] 6.1 Example 1: Affinity Maturation and Binding Affinity Studies of Humanized 08A Antibody
[0376] 6.1.1 Affinity maturation of 08A by single CDR codon-based mutagenesis library
[0377] CDRH3, CDRL1 and CDRL3 of humanized 08A (with S61N glycosylation site correction, numbered according to SEQ ID sequence) Fab001 (SEQ ID NOs: 1 and 2) were targeted for codon-based mutagenesis. The H3, L1 and L3 libraries were randomized at positions H95-H100B, L27B-L32 and L90-L97, respectively. Sequencing of a representative single colony from each library confirmed randomization in the expected CDRs of the targeted residues. Each library yields more than 10 8 a single colony, indicating that the size of the library is sufficient to account for every possible combination of amino acids within the target region.
[0378] Libraries were subjected to four rounds of affinity-based solution-phase phage display selection, each round with decreasing antigen concentration. The first round ...
Embodiment 2
[0405] 6.2 Example 2: Affinity of mouse and humanized anti-PD-1 mAbs to human PD1
[0406] 6.2.1 Surface kinetics determined by BIAcore
[0407] Surface plasmon resonance (SPR)-based assays using capture mode were used to determine the binding kinetics and affinity of anti-PD-1 antibodies to polyhistidine-tagged human PD-1 (hPD1-His, 98AFK). The S-series sensor chip CM5 (GE Healthcare, BR100530) was activated using an amine coupling kit (GE Healthcare, BR100050) according to the manufacturer's instructions. Human capture kit (anti-human Fc, 25 μg / mL, GE Healthcare, BR100839) or mouse capture kit (anti-mouse Fc, 30 μg / mL, GE Healthcare) diluted in provided pH 5.0, 10 mM sodium acetate buffer , BR100838) antibody was immobilized on the activated surface for 7 min. After fixation, the surface was inactivated with 1M ethanolamine / HCl (pH 8.5) for 7 minutes. In each of the four flow cells, final immobilization levels reached -8,000 resonance units (RU) for mouse capture and -12,...
Embodiment 3
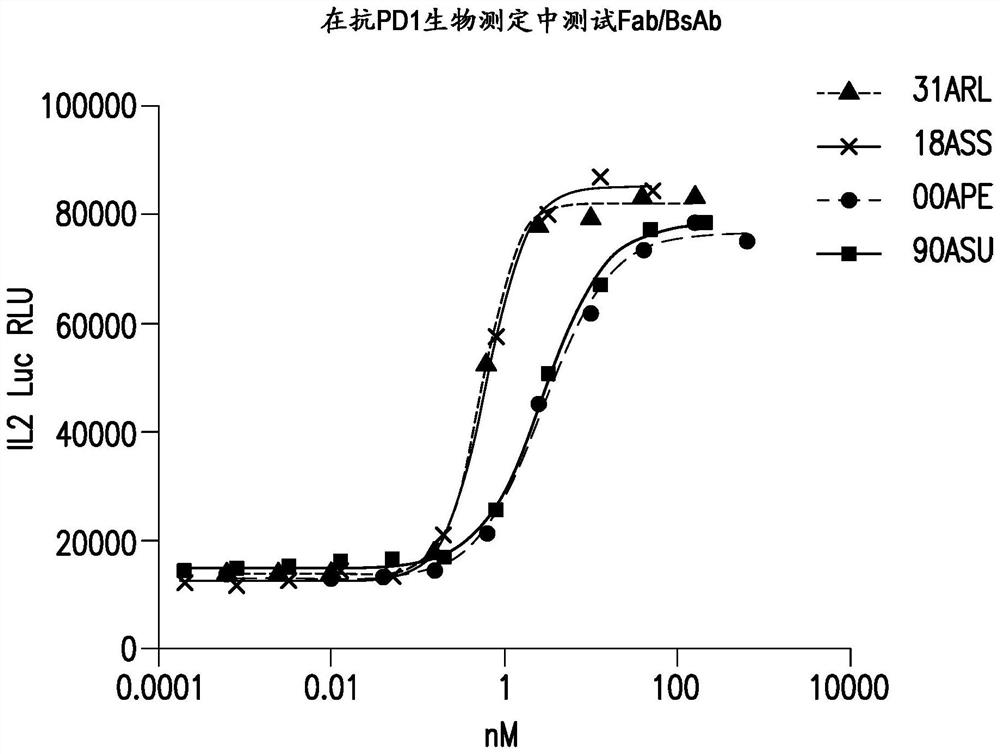
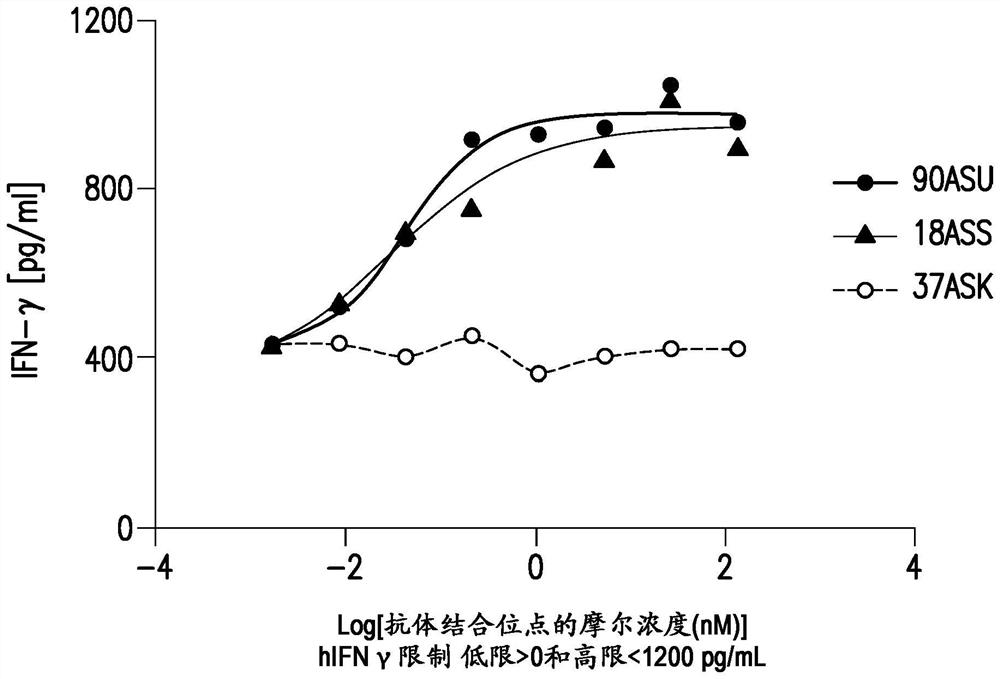
[0422] 6.3 Example 3: Generation of anti-PD-1 / LAG3 bispecific antibodies
[0423] Anti-PD-1 / LAG-3 bispecific antibody (BsAb) 18ASS has an anti-PD-1 heavy chain with the heavy chain variable region of affinity matured Fab100 (with CDRH2 S61N and G56A corrections) and an IgG1 constant region (with CH1 mutations L145E, K147T, Q175E and S183L, CH2 mutations L234A, L235A and D265S, CH3 mutations T350V, L351Y, F405A, Y407V) (SEQ ID NO: 102); anti-PD-1 light chain with affinity matured Fab100 Light chain variable region and kappa constant region with Cκ mutations Q124R, T178R (SEQ ID NO: 103); anti-LAG3 heavy chain with heavy chain variable region and IgG1 constant region of humanized 22D2 antibody Ab6 of WO 2016028672 ( With CH1 mutation S181K, CH2 mutation L234A, L235A and D265S, CH3 mutation T350V, T366L, K392L and T394W) (SEQ ID NO: 96); anti-LAG3 light chain, light chain variable region and kappa constant of antibody Ab6 of WO2016028672 Region (with CK mutations Q124E, S131T, T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com