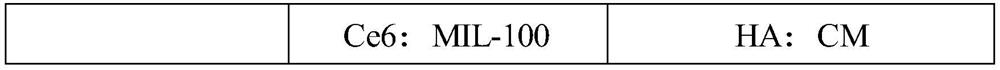Nano-particle for mediating cascade reaction, and preparation method thereof
A nanoparticle and cascade reaction technology, which can be used in medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulations, etc., can solve the problems that the therapeutic effect needs to be improved, and the tumor is destroyed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a method for preparing nanoparticles that mediate cascade reactions described in the above technical solution, comprising the following steps:
[0042] a) After mixing metal-organic framework MIL-100 and chlorin e6 with a solvent, centrifuge to obtain metal-organic framework MIL-100 particles loaded with chlorin e6;
[0043] b) Mixing the metal organic framework MIL-100 particles loaded with chlorin e6 with hyaluronic acid and water, and centrifuging to obtain nanoparticles.
[0044] Regarding step a):
[0045] In the present invention, the mass ratio of the metal organic framework MIL-100 to chlorin e6 is preferably (0.5-3):(0.5-3), more preferably (0.5-2):(0.5-2). In some embodiments of the present invention, the mass ratio is 1:(0.5-2).
[0046] In the present invention, the solvent is preferably one or more of dimethyl sulfoxide (ie DMSO), N,N-dimethylformamide (ie DMF) and ethanol. The ratio of the metal-organic framework MIL-...
Embodiment 1~9
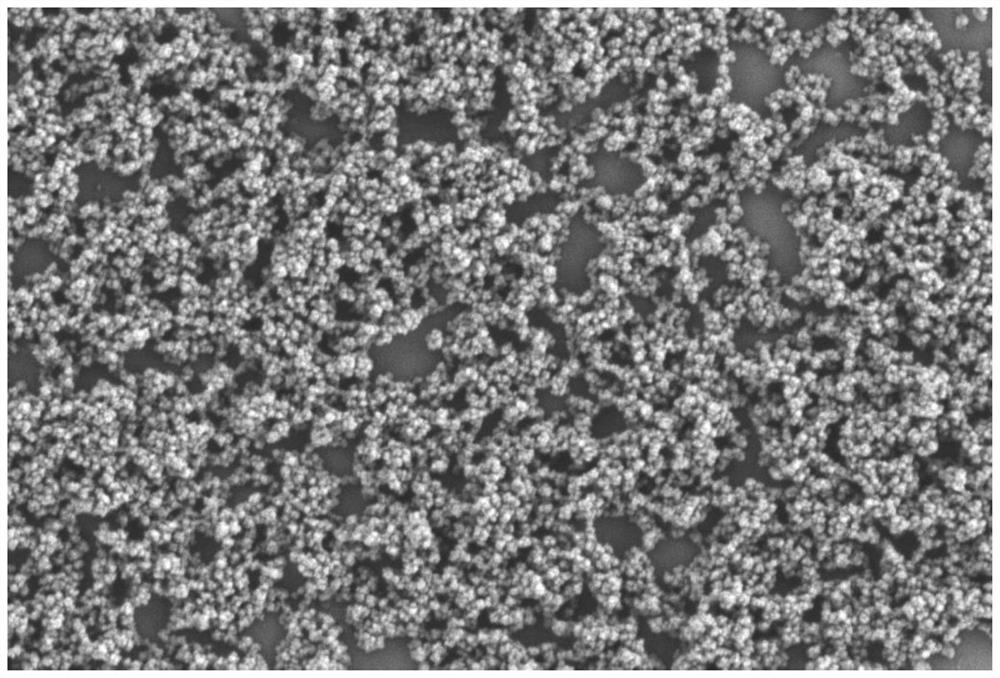
[0074] Preparation of S1, MIL-100:
[0075] Add ferric chloride hexahydrate and trimesic acid in a molar ratio of 2.25:1 into the DMF solvent (the ratio of the total mass of the reaction raw materials to the solvent is 1.12g:10mL), control the microwave reaction temperature at 130°C, and react for 5 minutes to obtain Metal organic framework material MIL-100.
[0076] S2, the preparation of nanoparticles:
[0077] The metal organic framework materials MIL-100 and Ce6 were mixed and stirred with the solvent DMSO (mass ratio of Ce6:MIL-100=0.5-2:1; the total volume of materials in the system was 8mL) at a stirring rate of 300rpm for 12h. Afterwards, the product was collected by centrifugation at a centrifugation rate of 8000 rpm and a centrifugation time of 10 min to obtain Ce6-loaded MIL-100 (denoted as CM).
[0078] The obtained CM, hyaluronic acid (HA, molecular weight 40000Da) and water were mixed and stirred (the mass ratio of HA: CM = 0.5-2: 1; the total volume of materials...
Embodiment 10
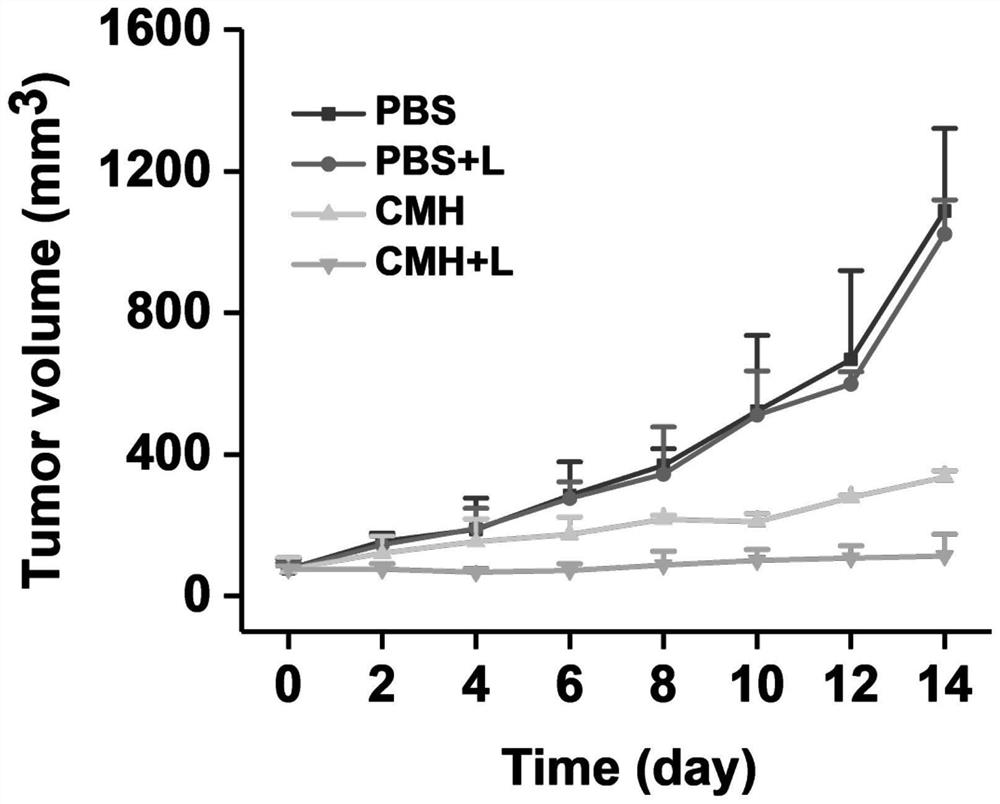
[0085] In vivo biodistribution assay
[0086] The experimental process is as follows: select about 20 g of Balb / C mice, and before tumor inoculation, take 4T1 cells in the logarithmic growth phase, and use 5×10 cells per mouse. 6 The density of the cells was inoculated subcutaneously on the outer side of the right hind limb of the mouse, and the tumor volume grew to 200-500mm 3 , the nanoparticles obtained in Example 2 were injected into the mice through the tail vein, and at different time points, the hearts, liver, spleen, lungs, kidneys and tumors of the mice were dissected out, and the organs and tumors were detected by fluorescence imaging equipment. The fluorescent signal is detected.
[0087] The experimental results showed that within 12 hours, with the prolongation of the injection time, the fluorescence signal of the tumor gradually increased. This is because the nanoparticles have a suitable size, can be enriched in tumor sites through the EPR effect and have the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com