Selective agonist of dopamine D2 receptor (DRD2) and application thereof in treatment of diseases
A technology of precursors and isomers, applied in the field of discovery of dopamine D2 receptor selective agonists, can solve problems such as hallucinations and movement disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0154] The present invention also provides a method for preparing a pharmaceutical composition or preparation for treating low dopamine-related diseases, the method comprising: mixing a therapeutically effective amount of a compound of formula I with a pharmaceutically acceptable carrier to form a pharmaceutical composition or preparation.
[0155] Typically, administering a therapeutically effective amount of a compound of formula I, or a pharmaceutical composition or preparation comprising a compound of formula I, to a subject can treat or alleviate low dopamine-related diseases (eg, Parkinson's).
[0156] DRD2 selective agonists and uses thereof
[0157] The invention provides a DRD2 selective agonist.
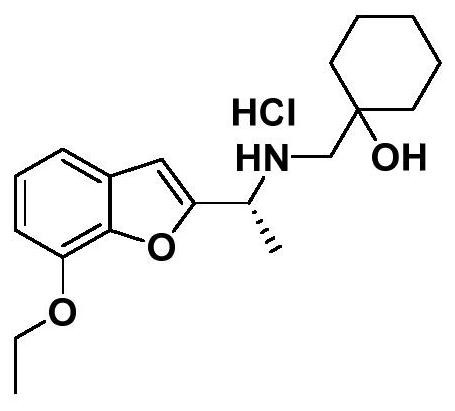
[0158] In a preferred example of the present invention, based on the complex structure of DRD2 and the antagonist haloperidol, the inventors continuously optimized the design and synthesized a DRD2 selective agonist by chemical method.
[0159] In another preferred exampl...
Embodiment 1
[0181] Embodiment 1 A kind of dopamine D 2 Discovery and Characterization of Receptor Selective Agonists
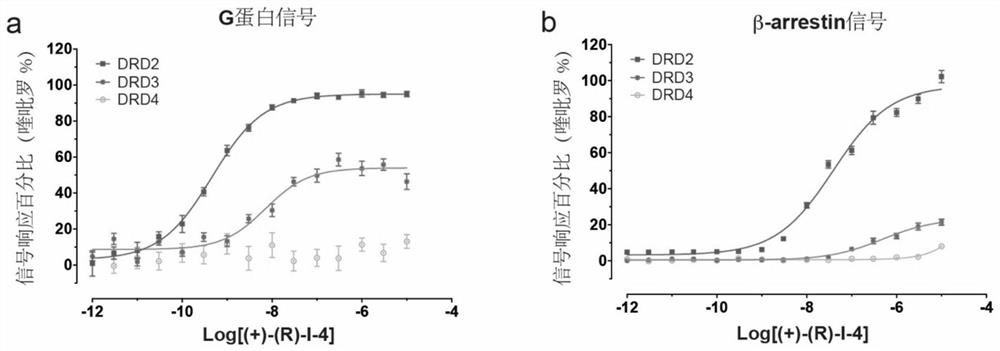
[0182] Inventor based on D 2 Based on the structural information of the receptor, a DRD2 selective agonist ( figure 1 ). The inventors selected two signaling pathways downstream of the receptor, the G protein signaling pathway and the β-arrestin signaling pathway, as indicators of the degree of receptor agonism, and verified their selectivity at the cellular level.
[0183] result( figure 2 ) shows that the performance of this agonist in the two signaling pathways is similar, whether it is for the activation of the G protein signaling pathway downstream of the dopamine receptor or for the activation of the β-arrestin signaling pathway under the dopamine receptor, the agonist has the same effect. Cannot activate DRD4, partially activate DRD3, fully activate DRD2, and the EC when this agonist plays a role in DRD3 50The value is about 15 times that of DRD2, which shows...
Embodiment 2
[0184] Embodiment 2 dopamine D 2 Application of receptor selective agonists in the treatment of animal models of Parkinson's disease
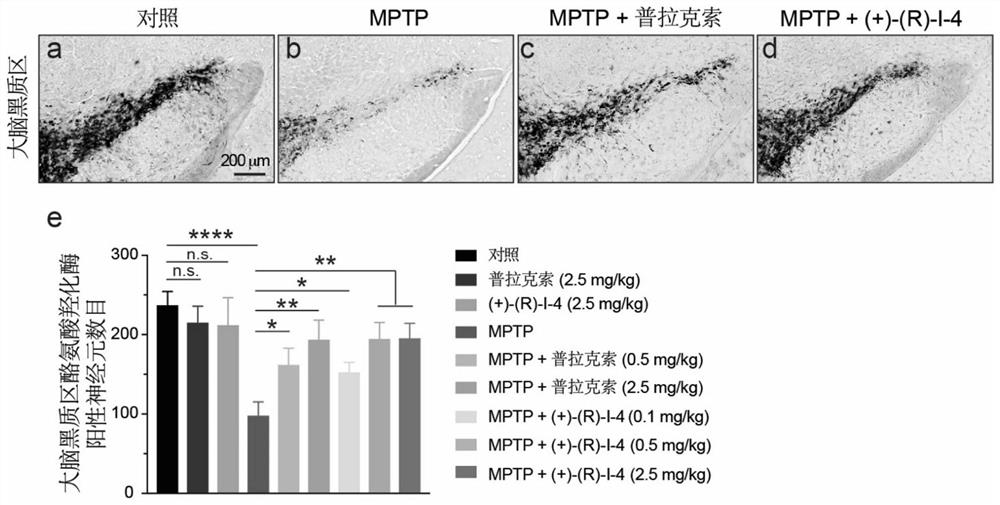
[0185] The inventors tested the neuroprotective effect of DRD2 agonist in the MPTP-induced Parkinson's disease mouse model, meanwhile, the Parkinson's disease drug pramipexole served as a positive control. result( image 3 ) showed that this agonist can relieve the MPTP-induced decrease in the expression of dopamine synthase tyrosine hydroxylase (TH) in the substantia nigra of the brain, revealing that it has a protective effect on dopaminergic neurons in the substantia nigra, And its effect is better than pramipexole (DRD2 / DRD3 / DRD4 agonist), which is currently clinically used to treat Parkinson's disease, which further suggests that this DRD2 agonist has the potential to treat Parkinson's disease more effectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com