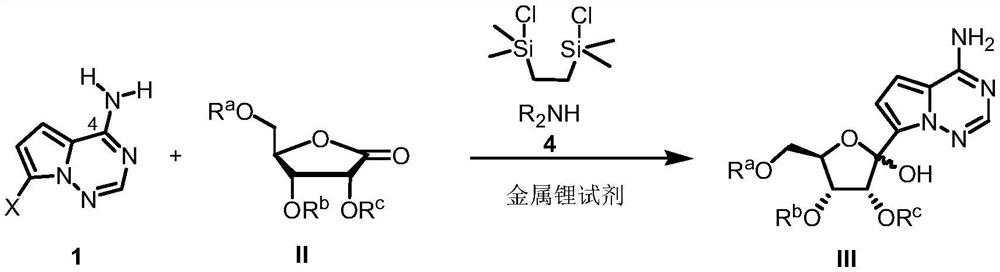
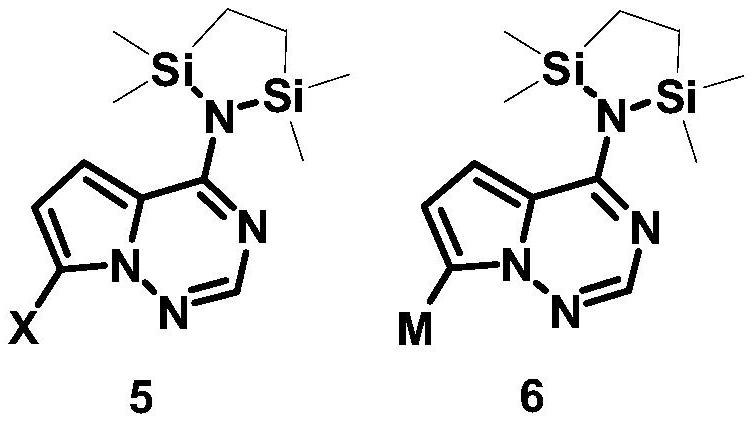
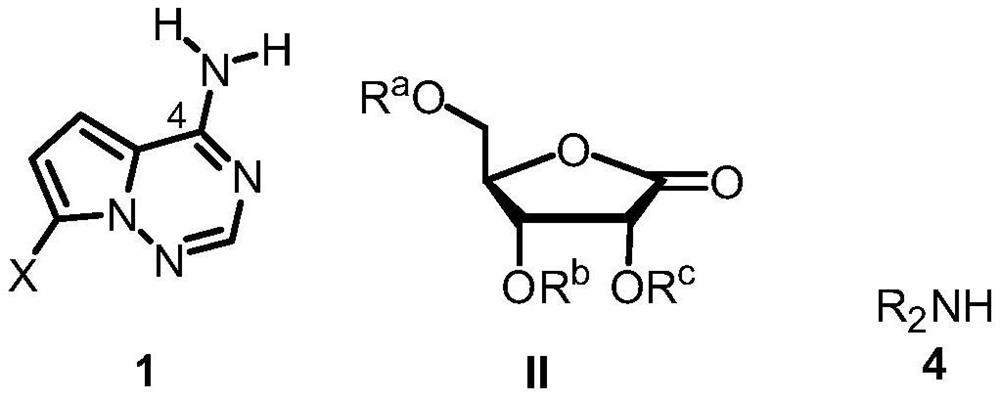
Synthetic method of C-nucleoside compound
A technology of nucleoside compounds and compounds, applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve the problems of difficult scale-up production, cumbersome operation, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] The synthesis of the chiral C-nucleoside compound shown in embodiment 1 formula 3
[0076]
[0077] Under anhydrous and oxygen-free conditions, after dissolving compound 1a (10.0g, 46.94mmol) and 1,2-bis(chlorodimethylsilyl)ethane (11.1g, 51.63mmol) in THF (100mL), add Diisopropylamine represented by Formula 4 (7.3 mL, 51.63 mmol). The reaction solution was sequentially added with n-butyllithium (81 mL, 201.8 mmol) and a THF solution (50 mL) of ribonolactone (39.3 g, 93.88 mmol) shown in formula 2 at -78°C. After the reaction solution was reacted at -78°C for 2 hours, citric acid aqueous solution (1M, 200mL) was added to quench the reaction. After the reaction solution returned to room temperature, the aqueous layer was extracted with ethyl acetate (3×200mL), the organic layers were combined, and water ( 1×250mL), saturated NaHCO 3 solution (1×250 mL), washed with saturated NaCl solution (1×250 mL). The organic layer was dried over anhydrous magnesium sulfate, fil...
Embodiment 2
[0079] According to the method described in Example 1, with the ribonolactone shown in compound 1a and formula 2 as substrates, the synthesis of chiral C-nucleoside compounds shown in the following formula 3 for different secondary amines shown in formula 4 The rates were compared and the data obtained are shown in the table below.
[0080]
[0081]
Embodiment 3
[0083] According to the method described in Example 1, compound 1a is reacted on a scale of 10 grams, and other reaction conditions are constant, only the concentration of compound 1a in the reaction solution and the equivalent number of ribonactone shown in formula 2 are changed, and the realized formula The data obtained for the synthesis yield of the chiral C-nucleoside compound shown in 3 are shown in the table below.
[0084]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com