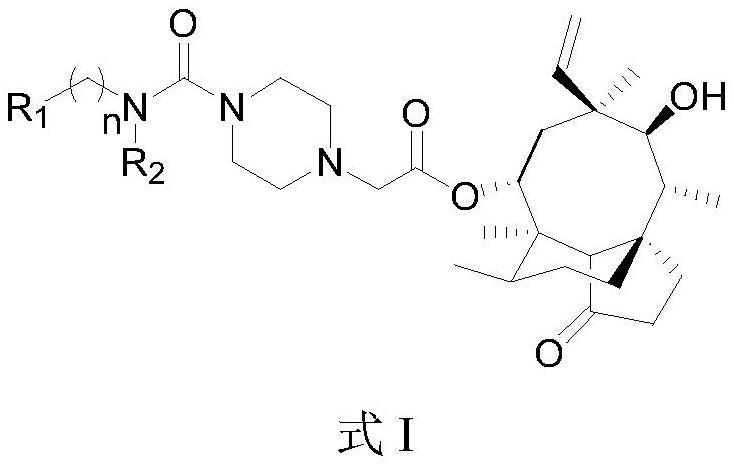
Piperazine urea pleuromutilin derivative and application thereof
A technology of pleuromutilin and its derivatives, applied in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve the problem that the antibacterial effect does not have much advantage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
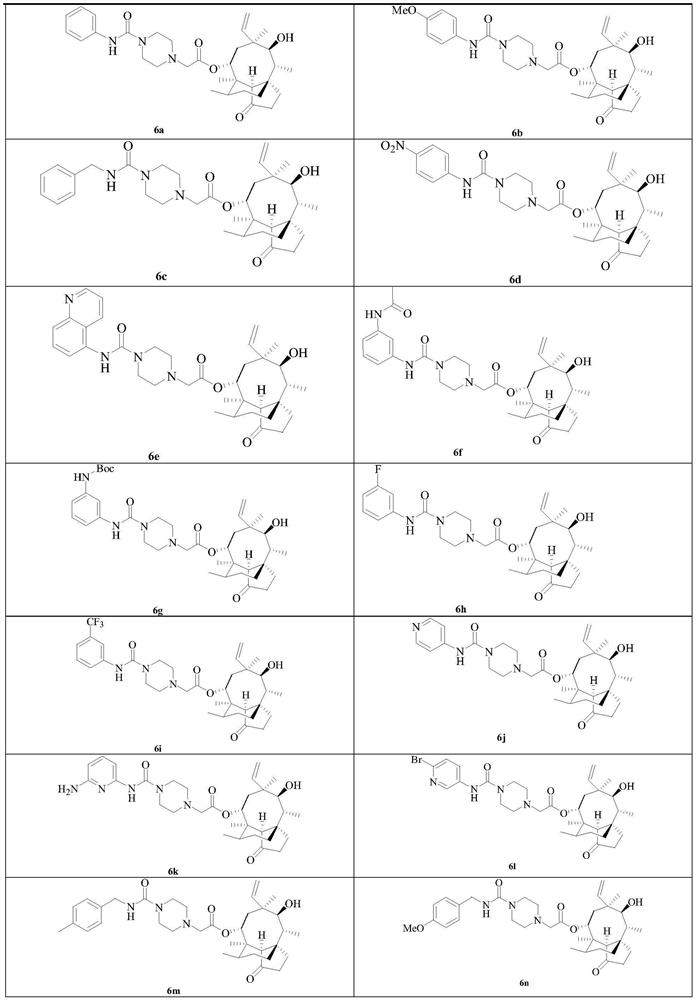
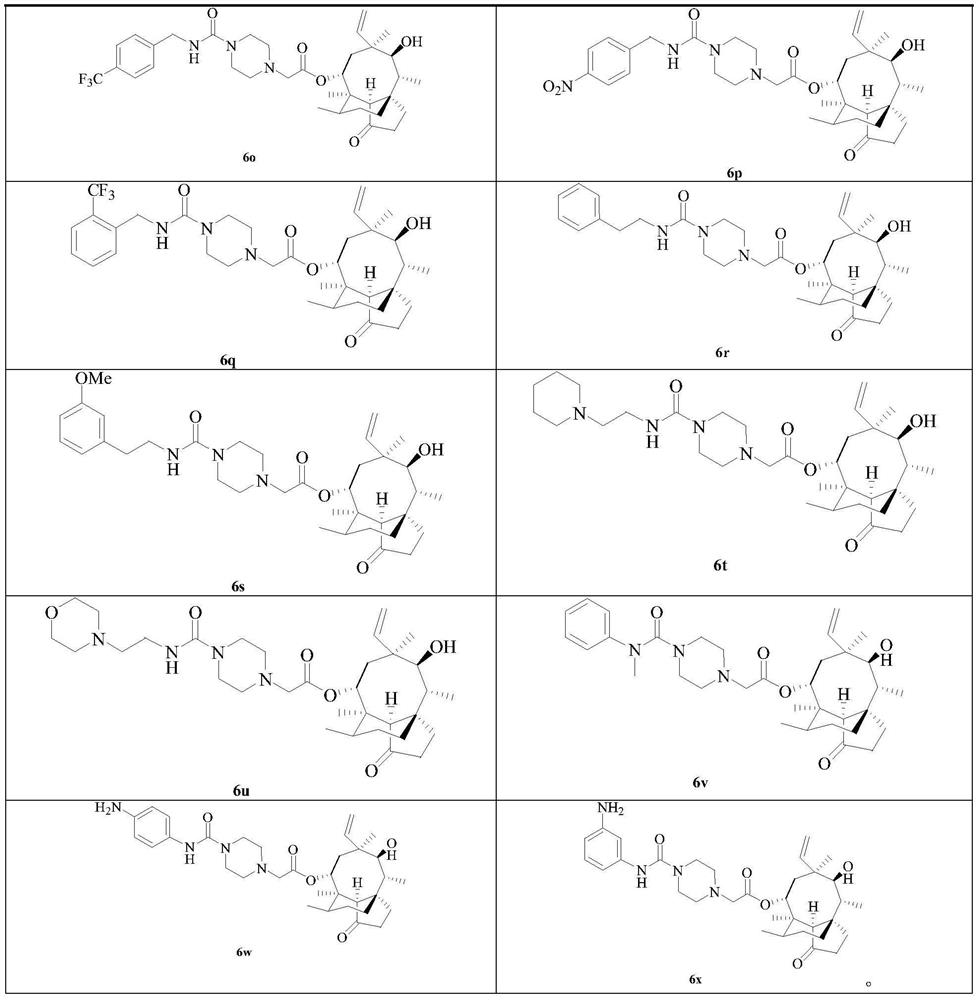
[0059] The synthesis of embodiment 1 compound 6a-v
[0060] Compounds 6a-v were synthesized according to the following synthetic route:
[0061]
[0062] Reagents and reaction conditions: (i) TsCl, triethylamine, DCM, 25°C; (ii) piperazine, K 2 CO 3 , NaI, THF, reflux; (iii) 9a~e, NaI, K 2 CO 3 ,MeCN,70℃; (iv)10f~u,K 2 CO 3 ,DMF,80℃; (v)11,DMAP,MeCN,70℃; (vi)SnCl 2 , EtOH, reflux; (vii) TFA, DCM, 25°C; (viii) (1) CDI, triethylamine, N-Bocpiperazine, MeCN and DMF, 25°C for 9a and 9b, or BTC, triethylamine, N-Boc Piperazine, THF and DCM, 25°C for 9c~e, (2) TFA, DCM, 25°C; (ix) Trichloroacetyl chloride, triethylamine, DCM, 25°C; (x) 4-nitrophenyl carbon Acid chloride, NMM, DCM, 25℃.
[0063] Synthesis of Compound 7:
[0064] Dissolve p-toluenesulfonyl chloride (1.91g, 10.0mmol) and pleuromutilin (3.56g, 9.4mmol) in DCM (10mL), then add triethylamine (4.54g, 12.0mmol), and react at room temperature for 20h. After the reaction was completed, water (50mL) was added, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com