A kind of ph-regulated self-assembled antimicrobial peptide and its preparation method and application
An antibacterial peptide, self-assembly technology, applied in the direction of antibacterial drugs, chemical instruments and methods, peptides, etc., can solve the problems of low biocompatibility and low stability of natural antibacterial peptides, which limit the application, and achieve low toxicity and good antibacterial properties. effect, good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the design of antibacterial peptide
[0016] Design principle: In order to obtain antibacterial peptides that can effectively kill pathogenic bacteria and have good biocompatibility, a single peptide molecule is used to form a supramolecular nanostructure through non-covalent self-assembly, and its antibacterial activity and biocompatibility are tested. Through detection, a self-assembled antimicrobial peptide with high application value was obtained. Among the twenty kinds of amino acids that constitute natural proteins, except for Gly, the α-carbon atoms of other protein amino acids are all achiral carbon atoms. Due to the different side chains of amino acids, they can provide different interaction forces for stabilizing the three-dimensional structure of proteins, such as hydrophobic forces, electrostatic forces and hydrogen bonds, etc., to achieve the stability of protein stereostructure chemistry and physical chemistry, and to achieve the minimum free...
Embodiment 2
[0021] Example 2: Preparation of assembled peptides
[0022] 1. Polypeptides were synthesized by Fmoc solid-phase synthesis method and freeze-dried. The purity of the product was determined by electrospray mass spectrometry (ESI-MS) and reversed-phase high-performance liquid chromatography (RP-HPLC), and the purity of the antimicrobial peptide was greater than 95%.
[0023] 2. Completely dissolve the freeze-dried antimicrobial peptide powder that has passed the quality inspection in ultrapure water with pH = 6.0, sonicate for 30 minutes, and place it at room temperature for 6-7 days to allow the peptide molecules to self-assemble into nanoparticles.
Embodiment 3
[0025] 1. Morphological observation of assembled peptides
[0026] Apply 10 μl of peptide solution on a copper grid (400 square mesh), absorb for 5 minutes, then stain with 0.1% phosphotungstic acid for 30 seconds, dry for 15 minutes, and observe with a microscope. The result is as Figure 1 As shown, the self-assembling peptide SAP forms spherical nanostructures in an acidic environment.
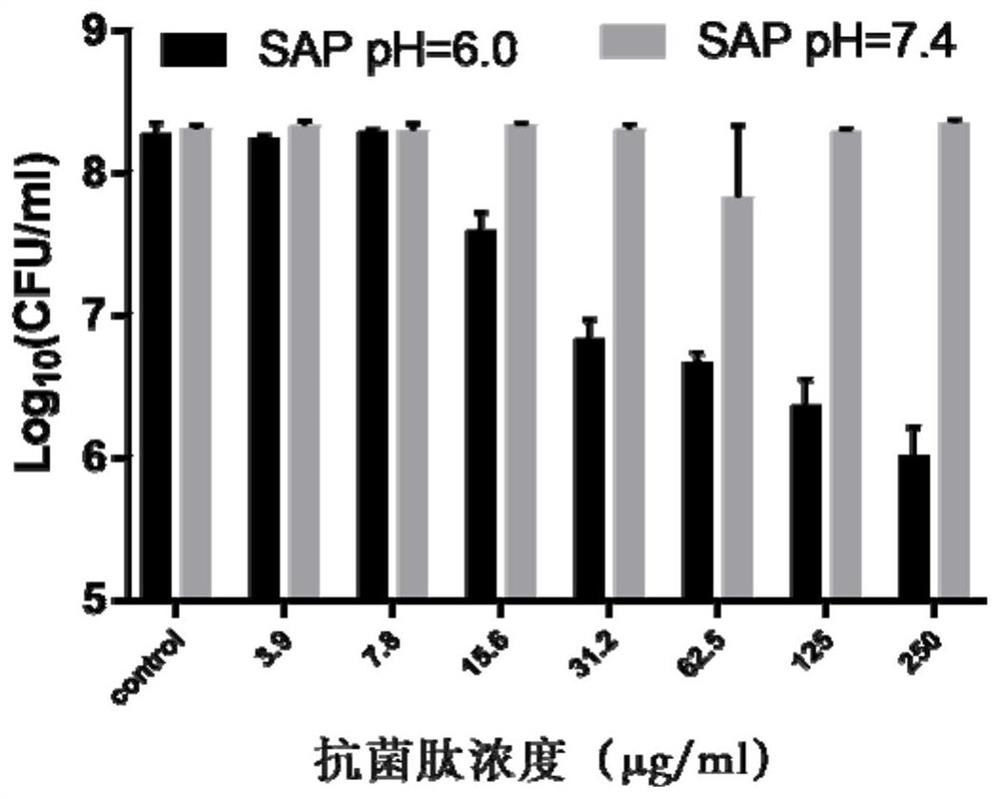
[0027] 2. Determination of Antimicrobial Peptide Activity
[0028] A single colony was isolated from the agar plate and cultured in 5 ml of 3% tryptic soy broth (TSB) medium with shaking at 200 rpm overnight at 37°C. Add 50 μl of bacterial solution to 5 ml of fresh 3% TSB, shake at 200 rpm, and cultivate to logarithmic phase. OD 600 The bacterial solution of =0.4 was diluted 1000 times, treated with different concentrations of peptides, incubated at 37°C for 3 hours, then serially diluted the bacterial solution, inoculated on TSB agar, and incubated overnight at 37°C. Count the number ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com